Identifying genetic networks underlying myometrial transition to labor
- PMID: 15693941
- PMCID: PMC551532
- DOI: 10.1186/gb-2005-6-2-r12
Identifying genetic networks underlying myometrial transition to labor
Abstract
Background: Early transition to labor remains a major cause of infant mortality, yet the causes are largely unknown. Although several marker genes have been identified, little is known about the underlying global gene expression patterns and pathways that orchestrate these striking changes.
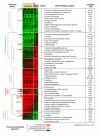
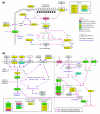
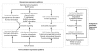
Results: We performed a detailed time-course study of over 9,000 genes in mouse myometrium at defined physiological states: non-pregnant, mid-gestation, late gestation, and postpartum. This dataset allowed us to identify distinct patterns of gene expression that correspond to phases of myometrial 'quiescence', 'term activation', and 'postpartum involution'. Using recently developed functional mapping tools (HOPACH (hierarchical ordered partitioning and collapsing hybrid) and GenMAPP 2.0), we have identified new potential transcriptional regulatory gene networks mediating the transition from quiescence to term activation.
Conclusions: These results implicate the myometrium as an essential regulator of endocrine hormone (cortisol and progesterone synthesis) and signaling pathways (cyclic AMP and cyclic GMP stimulation) that direct quiescence via the transcriptional upregulation of both novel and previously associated regulators. With term activation, we observe the upregulation of cytoskeletal remodeling mediators (intermediate filaments), cell junctions, transcriptional regulators, and the coordinate downregulation of negative control checkpoints of smooth muscle contractile signaling. This analysis provides new evidence of multiple parallel mechanisms of uterine contractile regulation and presents new putative targets for regulating myometrial transformation and contraction.
Figures

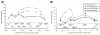
Similar articles
-
Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour.Eur J Obstet Gynecol Reprod Biol. 2009 May;144 Suppl 1:S2-10. doi: 10.1016/j.ejogrb.2009.02.044. Epub 2009 Mar 18. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19299064 Review.
-
The focal adhesion protein Hic-5 is highly expressed in the rat myometrium during late pregnancy and labour and co-localizes with FAK.Reprod Biol Endocrinol. 2007 Jun 5;5:22. doi: 10.1186/1477-7827-5-22. Reprod Biol Endocrinol. 2007. PMID: 17550607 Free PMC article.
-
Pregnancy and estradiol modulate myometrial G-protein pathways in the guinea pig.Am J Obstet Gynecol. 2006 Jul;195(1):275-87. doi: 10.1016/j.ajog.2005.12.050. Epub 2006 May 8. Am J Obstet Gynecol. 2006. PMID: 16681987
-
Human myometrial quiescence and activation during gestation and parturition involve dramatic changes in expression and activity of particulate type II (RII alpha) protein kinase A holoenzyme.J Clin Endocrinol Metab. 2003 May;88(5):2194-205. doi: 10.1210/jc.2002-021862. J Clin Endocrinol Metab. 2003. PMID: 12727975
-
Cyclic AMP signalling pathways in the regulation of uterine relaxation.BMC Pregnancy Childbirth. 2007 Jun 1;7 Suppl 1(Suppl 1):S10. doi: 10.1186/1471-2393-7-S1-S10. BMC Pregnancy Childbirth. 2007. PMID: 17570154 Free PMC article. Review.
Cited by
-
Analysis of two birth tissues provides new insights into the epigenetic landscape of neonates born preterm.Clin Epigenetics. 2019 Feb 11;11(1):26. doi: 10.1186/s13148-018-0599-4. Clin Epigenetics. 2019. PMID: 30744680 Free PMC article.
-
Alternative splicing regulates mouse embryonic stem cell pluripotency and differentiation.Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10514-9. doi: 10.1073/pnas.0912260107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498046 Free PMC article.
-
Pathways and genes differentially expressed in the motor cortex of patients with sporadic amyotrophic lateral sclerosis.BMC Genomics. 2007 Jan 23;8:26. doi: 10.1186/1471-2164-8-26. BMC Genomics. 2007. PMID: 17244347 Free PMC article.
-
An evolutionary conserved role for anaplastic lymphoma kinase in behavioral responses to ethanol.PLoS One. 2011;6(7):e22636. doi: 10.1371/journal.pone.0022636. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799923 Free PMC article.
-
Human effector/initiator gene sets that regulate myometrial contractility during term and preterm labor.Am J Obstet Gynecol. 2010 May;202(5):474.e1-20. doi: 10.1016/j.ajog.2010.02.034. Am J Obstet Gynecol. 2010. PMID: 20452493 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

