Polarized monocyte response to cytokine stimulation
- PMID: 15693944
- PMCID: PMC551535
- DOI: 10.1186/gb-2005-6-2-r15
Polarized monocyte response to cytokine stimulation
Abstract
Background: Mononuclear phagocytes (MPs) stand at the crossroads between the induction of acute inflammation to recruit and activate immune effector cells and the downmodulation of the inflammatory process to contain collateral damage. This decision is extensively modulated by the cytokine microenvironment, which includes a broad array of cytokines whose direct effect on MPs remains largely unexplored. Therefore, we tested whether polarized responses of MPs to pathogens are related to the influence of selected cytokines or represent a mandatory molecular switch through which most cytokines operate.
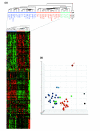
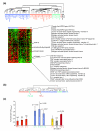
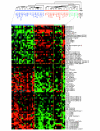
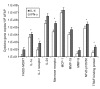
Results: Circulating CD14+ MPs were exposed to bacterial lipopolysaccharide (LPS) followed by exposure to an array of cytokines, chemokines and soluble factors involved in the immune response. Gene expression was studied by global transcript analysis. Two main classes of cytokines were identified that induced a classical or an alternative pathway of MP activation. Expression of genes affected by NFkappaB activation was most predictive of the two main classes, suggesting that this pathway is a fundamental target of cytokine regulation. As LPS itself induces a classical type of activation, the most dramatic modulation was observed toward the alternative pathway, suggesting that a broad array of cytokines may counteract the pro-inflammatory effects of bacterial components.
Conclusions: This analysis is directly informative of the primary effect of individual cytokines on the early stages of LPS stimulation and, therefore, may be most informative of the way MP maturation may be polarized at the early stages of the immune response.
Figures


Similar articles
-
Transcriptional analysis of LPS-stimulated activation of trout (Oncorhynchus mykiss) monocyte/macrophage cells in primary culture treated with cortisol.Mol Immunol. 2006 Mar;43(9):1340-8. doi: 10.1016/j.molimm.2005.09.005. Epub 2005 Oct 18. Mol Immunol. 2006. PMID: 16239032
-
Ex vivo effects of high-density lipoprotein exposure on the lipopolysaccharide-induced inflammatory response in patients with severe cirrhosis.Hepatology. 2009 Jan;49(1):175-84. doi: 10.1002/hep.22582. Hepatology. 2009. PMID: 19053046
-
Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes.J Immunol. 1993 Dec 15;151(12):6692-700. J Immunol. 1993. PMID: 8258685
-
Interferon-gamma modulates the lipopolysaccharide-induced expression of AP-1 and NF-kappa B at the mRNA and protein level in human monocytes.Exp Hematol. 1996 Feb;24(2):228-35. Exp Hematol. 1996. PMID: 8641346
-
Cellular immune and cytokine pathways resulting in tissue factor expression and relevance to septic shock.Nouv Rev Fr Hematol (1978). 1992;34 Suppl:S15-27. Nouv Rev Fr Hematol (1978). 1992. PMID: 1364116 Review.
Cited by
-
Molecular immune signatures of HIV-1 vaccines in human PBMCs.FEBS Lett. 2009 Sep 17;583(18):3004-8. doi: 10.1016/j.febslet.2009.07.060. Epub 2009 Aug 6. FEBS Lett. 2009. PMID: 19665024 Free PMC article.
-
Adaptation in human immune cells residing in tissues at the frontline of infections.Nat Commun. 2024 Nov 28;15(1):10329. doi: 10.1038/s41467-024-54603-5. Nat Commun. 2024. PMID: 39609395 Free PMC article.
-
Systemic treatment of xenografts with vaccinia virus GLV-1h68 reveals the immunologic facet of oncolytic therapy.BMC Genomics. 2009 Jul 7;10:301. doi: 10.1186/1471-2164-10-301. BMC Genomics. 2009. PMID: 19583830 Free PMC article.
-
MMP7 cleaves remyelination-impairing fibronectin aggregates and its expression is reduced in chronic multiple sclerosis lesions.Glia. 2018 Aug;66(8):1625-1643. doi: 10.1002/glia.23328. Epub 2018 Mar 30. Glia. 2018. PMID: 29600597 Free PMC article.
-
Blood classical monocytes phenotype is not altered in primary non-small cell lung cancer.World J Clin Oncol. 2014 Dec 10;5(5):1078-87. doi: 10.5306/wjco.v5.i5.1078. World J Clin Oncol. 2014. PMID: 25493244 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

