The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease
- PMID: 15693956
- PMCID: PMC551528
- DOI: 10.1186/gb-2005-6-2-209
The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease
Abstract
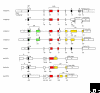
Vascular endothelial growth factors (VEGFs) are a family of secreted polypeptides with a highly conserved receptor-binding cystine-knot structure similar to that of the platelet-derived growth factors. VEGF-A, the founding member of the family, is highly conserved between animals as evolutionarily distant as fish and mammals. In vertebrates, VEGFs act through a family of cognate receptor kinases in endothelial cells to stimulate blood-vessel formation. VEGF-A has important roles in mammalian vascular development and in diseases involving abnormal growth of blood vessels; other VEGFs are also involved in the development of lymphatic vessels and disease-related angiogenesis. Invertebrate homologs of VEGFs and VEGF receptors have been identified in fly, nematode and jellyfish, where they function in developmental cell migration and neurogenesis. The existence of VEGF-like molecules and their receptors in simple invertebrates without a vascular system indicates that this family of growth factors emerged at a very early stage in the evolution of multicellular organisms to mediate primordial developmental functions.
Figures

References
-
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. The initial discovery of a secreted VPF with the characteristics of VEGF-A. - PubMed
-
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. This and [4] are the first reports of the cDNA cloning of VEGF-A. - PubMed
-
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. See [3]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

