Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination
- PMID: 15694005
- PMCID: PMC548684
- DOI: 10.1186/1471-2172-6-3
Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination
Abstract
Background: The function of CD57+ CD4+ T cells, constituting a major subset of germinal center T (GC-Th) cells in human lymphoid tissues, has been unclear. There have been contradictory reports regarding the B cell helping function of CD57+ GC-Th cells in production of immunoglobulin (Ig). Furthermore, the cytokine and co-stimulation requirement for their helper activity remains largely unknown. To clarify and gain more insight into their function in helping B cells, we systematically investigated the capacity of human tonsil CD57+ GC-Th cells in inducing B cell Ig synthesis.
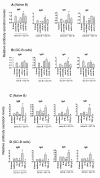
Results: We demonstrated that CD57+ GC-Th cells are highly efficient in helping B cell production of all four subsets of Ig (IgM, IgG, IgA and IgE) compared to other T-helper cells located in germinal centers or interfollicular areas. CD57+ GC-Th cells were particularly more efficient than other T cells in helping GC-B cells but not naive B cells. CD57+ GC-Th cells induced the expression of activation-induced cytosine deaminase (AID) and class switch recombination in developing B cells. IgG1-3 and IgA1 were the major Ig isotypes induced by CD57+ GC-Th cells. CD40L, but not IL-4, IL-10 and IFN-gamma, was critical in CD57+ GC-Th cell-driven B cell production of Ig. However, IL-10, when added exogenously, significantly enhanced the helper activity of CD57+ GC-Th cells, while TGF-beta1 completely and IFN-gamma partially suppressed the CD57+ GC-Th cell-driven Ig production.
Conclusions: CD57+CD4+ T cells in the germinal centers of human lymphoid tissues are the major T helper cell subset for GC-B cells in Ig synthesis. Their helper activity is consistent with their capacity to induce AID and class switch recombination, and can be regulated by CD40L, IL-4, IL-10 and TGF-beta.
Figures


Similar articles
-
Cytokine expression by germinal center cells.J Immunol. 1993 Jan 1;150(1):39-47. J Immunol. 1993. PMID: 7678034
-
Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression.Eur J Immunol. 2006 Jul;36(7):1892-903. doi: 10.1002/eji.200636136. Eur J Immunol. 2006. PMID: 16791882
-
Immunoglobulin production induced by CD57+ GC-derived helper T cells in vitro requires addition of exogenous IL-2.Cell Immunol. 1996 May 1;169(2):166-73. doi: 10.1006/cimm.1996.0107. Cell Immunol. 1996. PMID: 8620544
-
Memory B cells in mouse models.Scand J Immunol. 2013 Aug;78(2):149-56. doi: 10.1111/sji.12073. Scand J Immunol. 2013. PMID: 23679222 Review.
-
Germinal center structure and function: lessons from CD19.Semin Immunol. 2008 Feb;20(1):43-8. doi: 10.1016/j.smim.2007.12.007. Epub 2008 Feb 19. Semin Immunol. 2008. PMID: 18243730 Free PMC article. Review.
Cited by
-
T-cell phenotype including CD57+ T follicular helper cells in the tumor microenvironment correlate with a poor outcome in follicular lymphoma.Blood Cancer J. 2023 Aug 18;13(1):124. doi: 10.1038/s41408-023-00899-3. Blood Cancer J. 2023. PMID: 37591873 Free PMC article.
-
CD10 delineates a subset of human IL-4 producing follicular helper T cells involved in the survival of follicular lymphoma B cells.Blood. 2015 Apr 9;125(15):2381-5. doi: 10.1182/blood-2015-02-625152. Epub 2015 Mar 2. Blood. 2015. PMID: 25733581 Free PMC article.
-
The good, the bad and the ugly - TFH cells in human health and disease.Nat Rev Immunol. 2013 Jun;13(6):412-26. doi: 10.1038/nri3447. Epub 2013 May 17. Nat Rev Immunol. 2013. PMID: 23681096 Review.
-
Quantitative Multiplexed Imaging Analysis Reveals a Strong Association between Immunogen-Specific B Cell Responses and Tonsillar Germinal Center Immune Dynamics in Children after Influenza Vaccination.J Immunol. 2018 Jan 15;200(2):538-550. doi: 10.4049/jimmunol.1701312. Epub 2017 Dec 13. J Immunol. 2018. PMID: 29237774 Free PMC article.
-
Focal lymphocytic sialadenitis and ectopic germinal centers in oral reactive lesions and primary Sjögren's syndrome: a comparative study.Rheumatol Int. 2022 Aug;42(8):1411-1421. doi: 10.1007/s00296-021-04949-6. Epub 2021 Jul 20. Rheumatol Int. 2022. PMID: 34283264
References
-
- Ziegner M, Steinhauser G, Berek C. Development of antibody diversity in single germinal centers: selective expansion of high-affinity variants. Eur J Immunol. 1994;24:2393–2400. - PubMed
-
- Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-G. - DOI - PubMed
-
- Jumper MD, Splawski JB, Lipsky PE, Meek K. Ligation of CD40 induces sterile transcripts of multiple Ig H chain isotypes in human B cells. J Immunol. 1994;152:438–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

