Post-tetanic potentiation in the rat calyx of Held synapse
- PMID: 15695246
- PMCID: PMC1456049
- DOI: 10.1113/jphysiol.2004.079160
Post-tetanic potentiation in the rat calyx of Held synapse
Abstract
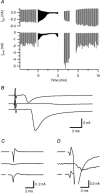
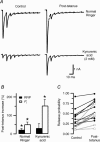
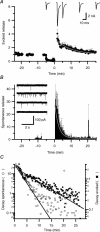
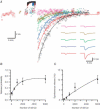
We studied synaptic plasticity in the calyx of Held synapse, an axosomatic synapse in the auditory brainstem, by making whole-cell patch clamp recordings of the principal cells innervated by the calyces in a slice preparation of 7- to 10-day-old rats. A 5 min 20 Hz stimulus train increased the amplitude of excitatory postsynaptic currents (EPSCs) on average more than twofold. The amplitude of the synaptic currents took several minutes to return to control values. The post-tetanic potentiation (PTP) was accompanied by a clear increase in the frequency, but not the amplitude, of spontaneous EPSCs, which returned to baseline more rapidly than the potentiation of evoked release. The size of the readily releasable pool of vesicles was increased by about 30%. In experiments in which presynaptic measurements of the intracellular calcium concentration were combined with postsynaptic voltage clamp recordings, PTP was accompanied by an increase in the presynaptic calcium concentration to about 210 nM. The decay of the PTP matched the decay of this increase. When the decay of the calcium transient was shortened by dialysing the terminal with EGTA, the PTP decay sped up in parallel. Our experiments suggest that PTP at the calyx of Held synapse is due to a long-lasting increase in the presynaptic calcium concentration following a tetanus, which results in an increase in the release probability of the vesicles of the readily releasable pool. Although part of the PTP can be explained by a direct activation of the calcium sensor for phasic release, other mechanisms are likely to contribute as well.
Figures


Similar articles
-
An increase in calcium influx contributes to post-tetanic potentiation at the rat calyx of Held synapse.J Neurophysiol. 2006 Dec;96(6):2868-76. doi: 10.1152/jn.00427.2006. Epub 2006 Aug 9. J Neurophysiol. 2006. PMID: 16899643
-
Presynaptic Ca2+ requirements and developmental regulation of posttetanic potentiation at the calyx of Held.J Neurosci. 2005 May 25;25(21):5127-37. doi: 10.1523/JNEUROSCI.1295-05.2005. J Neurosci. 2005. PMID: 15917453 Free PMC article.
-
Dynamics of the readily releasable pool during post-tetanic potentiation in the rat calyx of Held synapse.J Physiol. 2007 Jun 1;581(Pt 2):467-78. doi: 10.1113/jphysiol.2006.127365. Epub 2007 Mar 15. J Physiol. 2007. PMID: 17363387 Free PMC article.
-
The calyx of Held synapse: from model synapse to auditory relay.Annu Rev Physiol. 2012;74:199-224. doi: 10.1146/annurev-physiol-020911-153236. Epub 2011 Oct 24. Annu Rev Physiol. 2012. PMID: 22035348 Review.
-
Short-term plasticity at the calyx of Held.Nat Rev Neurosci. 2002 Jan;3(1):53-64. doi: 10.1038/nrn705. Nat Rev Neurosci. 2002. PMID: 11823805 Review.
Cited by
-
Molecular mechanisms governing Ca(2+) regulation of evoked and spontaneous release.Nat Neurosci. 2015 Jul;18(7):935-41. doi: 10.1038/nn.4044. Nat Neurosci. 2015. PMID: 26108721 Review.
-
Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission.J Neurosci. 2012 Aug 15;32(33):11441-52. doi: 10.1523/JNEUROSCI.1283-12.2012. J Neurosci. 2012. PMID: 22895726 Free PMC article.
-
Molecular Mechanisms of Short-Term Plasticity: Role of Synapsin Phosphorylation in Augmentation and Potentiation of Spontaneous Glutamate Release.Front Synaptic Neurosci. 2018 Oct 30;10:33. doi: 10.3389/fnsyn.2018.00033. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 30425632 Free PMC article.
-
Phosphorylation of synapsin domain A is required for post-tetanic potentiation.J Cell Sci. 2007 Sep 15;120(Pt 18):3228-37. doi: 10.1242/jcs.012005. Epub 2007 Aug 28. J Cell Sci. 2007. PMID: 17726061 Free PMC article.
-
Calcium-dependent isoforms of protein kinase C mediate posttetanic potentiation at the calyx of Held.Neuron. 2011 Jun 9;70(5):1005-19. doi: 10.1016/j.neuron.2011.04.019. Neuron. 2011. PMID: 21658591 Free PMC article.
References
-
- Angleson JK, Betz WJ. Intraterminal Ca2+ and spontaneous transmitter release at the frog neuromuscular junction. J Neurophysiol. 2001;85:287–294. - PubMed
-
- Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. - PubMed
-
- Bao JX, Kandel ER, Hawkins RD. Involvement of pre-and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science. 1997;275:969–973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

