Cellular stress failure in ventilator-injured lungs
- PMID: 15695492
- PMCID: PMC2718477
- DOI: 10.1164/rccm.200408-1036SO
Cellular stress failure in ventilator-injured lungs
Abstract
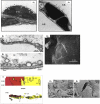
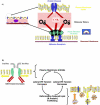
The clinical and experimental literature has unequivocally established that mechanical ventilation with large tidal volumes is injurious to the lung. However, uncertainty about the micromechanics of injured lungs and the numerous degrees of freedom in ventilator settings leave many unanswered questions about the biophysical determinants of lung injury. In this review we focus on experimental evidence for lung cells as injury targets and the relevance of these studies for human ventilator-associated lung injury. In vitro, the stress-induced mechanical interactions between matrix and adherent cells are important for cellular remodeling as a means for preventing compromise of cell structure and ultimately cell injury or death. In vivo, these same principles apply. Large tidal volume mechanical ventilation results in physical breaks in alveolar epithelial and endothelial plasma membrane integrity and subsequent triggering of proinflammatory signaling cascades resulting in the cytokine milieu and pathologic and physiologic findings of ventilator-associated lung injury. Importantly, though, alveolar cells possess cellular repair and remodeling mechanisms that in addition to protecting the stressed cell provide potential molecular targets for the prevention and treatment of ventilator-associated lung injury in the future.
Figures


References
-
- Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med 2003;31:S276–S284. - PubMed
-
- Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: An analysis of multiple cause mortality data (1979–1996). Crit Care Med 2002;30:1679–1685. - PubMed
-
- Bersten AD, Edibam C, Hunt T, Moran J. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med 2002;165:443–448. - PubMed
-
- Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983–1993. JAMA 1995;273:306–309. - PubMed
-
- Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frosell CG, Bonde J. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med 1999;159:1849–1861. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

