Convergent signaling by acidosis and receptor activator of NF-kappaB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts
- PMID: 15695591
- PMCID: PMC548977
- DOI: 10.1073/pnas.0406874102
Convergent signaling by acidosis and receptor activator of NF-kappaB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts
Abstract
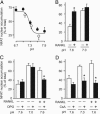
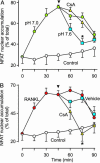
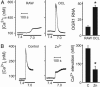
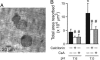
Systemic acidosis has detrimental effects on the skeleton, and local acidosis coincides with bone destruction in inflammatory and metastatic diseases. Acidification dramatically enhances osteoclastic resorption, although the underlying mechanism has remained elusive. We investigated the effect of acidosis on the osteoclastogenic transcription factor NFATc1, which upon dephosphorylation translocates from the cytoplasm to nuclei. Lowering extracellular pH dramatically increased accumulation of NFATc1 in nuclei of rat and rabbit osteoclasts to levels comparable with those induced by the proresorptive cytokine receptor activator of NF-kappaB ligand (RANKL). Activation of NFATc1 by RANKL was mediated by means of prolonged stimulation of the Ca2+/calmodulin-dependent protein phosphatase, calcineurin. In contrast, NFATc1 activation by acidosis involved stimulation of calcineurin and suppression of NFATc1 inactivation. Acidosis, like RANKL, induced transient elevation of cytosolic free Ca2+ concentration ([Ca2+]i), which persisted in Ca2+-free media and was abolished by inhibition of phospholipase C or depletion of intracellular Ca2+ stores. Real-time-PCR of osteoclast-like cells generated from RAW 264.7 cells revealed high levels of expression of ovarian cancer G protein-coupled receptor 1, which links extracellular acidification to elevation of [Ca2+]i. In addition, the calcineurin inhibitor cyclosporin A suppressed the stimulatory effect of acidification on resorption, implicating NFAT in mediating the actions of acidosis on osteoclast activity. In summary, acidification and RANKL induce signals in osteoclasts that converge on the Ca2+/calcineurin/NFAT pathway. Acidosis acts directly on osteoclasts to activate NFATc1 and stimulate resorption.
Figures


References
-
- Goto, K. (1918) J. Biol. Chem. 36, 355-376.
-
- Arnett, T. (2003) Proc. Nutr. Soc. 62, 511-520. - PubMed
-
- Bushinsky, D. A. (2001) Eur. J. Nutr. 40, 238-244. - PubMed
-
- Kraut, J. A., Mishler, D. R. & Kurokawa, K. (1984) Kidney Int. 25, 608-612. - PubMed
-
- Arnett, T. R. & Dempster, D. W. (1986) Endocrinology 119, 119-124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

