Iron-mediated control of the basic helix-loop-helix protein FER, a regulator of iron uptake in tomato
- PMID: 15695640
- PMCID: PMC1065402
- DOI: 10.1104/pp.104.054270
Iron-mediated control of the basic helix-loop-helix protein FER, a regulator of iron uptake in tomato
Abstract
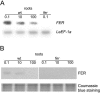
Root iron mobilization genes are induced by iron deficiency downstream of an unknown signaling mechanism. The FER gene, encoding a basic helix-loop-helix domain protein and putative transcription factor, is required for induction of iron mobilization genes in roots of tomato (Lycopersicon esculentum). To study upstream regulatory events of FER action, we examined the control of FER gene and FER protein expression in response to iron nutritional status. We analyzed expression of the FER gene and FER protein in wild-type plants, in mutant plants with defects in iron uptake regulation, and in 35S transgenic plants that overexpressed the FER gene. An affinity-purified antiserum directed against FER epitopes was produced that recognized FER protein in plant protein extracts. We found that the FER gene and FER protein were consistently down-regulated in roots after generous (100 mum, physiologically optimal) iron supply compared to low (0.1 mum) and sufficient (10 mum) iron supply. FER gene and FER protein expression were also occasionally down-regulated at sufficient compared to low iron supply. Analysis of FER protein expression in FER overexpression plants, as well as cellular protein localization studies, indicated that FER was down-regulated by high iron at the posttranscriptional level. The FER protein was targeted to plant nuclei and showed transcriptional activation in yeast (Saccharomyces cerevisiae). FER protein regulation in the iron accumulation mutant chloronerva indicated that FER protein expression was not directly controlled by signals derived from iron transport. We conclude that FER is able to affect transcription in the nucleus and its action is controlled by iron supply at multiple regulatory levels.
Figures





References
-
- Andrews NC, Fleming MD, Gunshin H (1999) Iron transport across biologic membranes. Nutr Rev 57: 114–123 - PubMed
-
- Bagg A, Neilands JB (1987) Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26: 5471–5477 - PubMed
-
- Bereczky Z, Wang HY, Schubert V, Ganal M, Bauer P (2003) Differential regulation of nramp and irt metal transporter genes in wild type and iron uptake mutants of tomato. J Biol Chem 278: 24697–24704 - PubMed
-
- Brown JC, Ambler JE (1974) Iron-stress response in tomato (Lycopersicon esculentum) 1. Sites of Fe reduction, absorption and transport. Physiol Plant 31: 221–224
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical

