Evaluation of two commercially available, inexpensive alternative assays used for assessing viral load in a cohort of human immunodeficiency virus type 1 subtype C-infected patients from South Africa
- PMID: 15695692
- PMCID: PMC548057
- DOI: 10.1128/JCM.43.2.857-861.2005
Evaluation of two commercially available, inexpensive alternative assays used for assessing viral load in a cohort of human immunodeficiency virus type 1 subtype C-infected patients from South Africa
Abstract
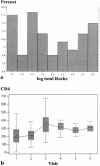
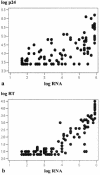
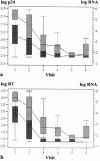
Although human immunodeficiency virus type 1 (HIV-1) RNA is the acknowledged "gold standard" marker for monitoring disease activity in patients receiving highly active antiretroviral therapy (HAART), it remains unaffordable in resource-constrained settings. The present study investigated two commercially available kits for the detection of HIV-1 viral load markers as more affordable alternatives to HIV-1 RNA quantitation. The greatly improved heat-denatured, signal-boosted HiSens HIV-1 p24 Ag Ultra kit (Perkin-Elmer) and the ExaVir Load Quantitative HIV-RT kit (Cavidi Tech AB) were compared with the Amplicor HIV-1 Monitor (version 1.5) assay (Roche Molecular Systems Inc.). A total of 117 samples containing HIV-1 subtype C were analyzed by all three methodologies. Eighty-nine of these samples represented serial measurements from 20 patients receiving HAART. The remaining samples analyzed were from a group of treatment-naive patients. The association between the p24 antigen assay and the RNA assay was fairly strong (R(2) = 0.686). The association between the reverse transcriptase (RT) quantitation assay and the RNA assay was strong (R(2) = 0.810). Both alternative assays seemed most useful for the serial monitoring of patients receiving HAART (n = 89 plasma samples from 20 patients), as all assays showed a statistically significant downward trend over time, with the trend being either linear or curvilinear. In addition, all three assays showed negative correlations with the CD4 count (CD4 count versus RNA load, r = -0.336 and P = 0.001; CD4 count versus p24 antigen level, r = -0.541 and P < 0.0001; CD4 count versus RT level, r = -0.358 and P = 0.0006). Still of major concern are both the lack of sensitivity and the wide degrees of variability of both assays. However, both assays provide a less expensive alternative to the Roche viral load assay and demonstrate the same trends during treatment.
Figures
Similar articles
-
Measurement of HIV-1 p24 antigen by signal-amplification-boosted ELISA of heat-denatured plasma is a simple and inexpensive alternative to tests for viral RNA.AIDS Rev. 2002 Apr-Jun;4(2):83-92. AIDS Rev. 2002. PMID: 12152521 Review.
-
Quantitation of human immunodeficiency virus type 1 viral load in plasma using reverse transcriptase activity assay at a district hospital laboratory in Botswana: a decentralization pilot study.J Virol Methods. 2009 Jul;159(1):93-7. doi: 10.1016/j.jviromet.2009.03.008. Epub 2009 Mar 20. J Virol Methods. 2009. PMID: 19442851
-
Evaluation of two commercially available alternatives for HIV-1 viral load testing in resource-limited settings.J Virol Methods. 2007 Dec;146(1-2):178-87. doi: 10.1016/j.jviromet.2007.06.019. Epub 2007 Aug 7. J Virol Methods. 2007. PMID: 17686534
-
Comparison of two human immunodeficiency virus (HIV) RNA surrogate assays to the standard HIV RNA assay.J Clin Microbiol. 2005 Dec;43(12):5950-6. doi: 10.1128/JCM.43.12.5950-5956.2005. J Clin Microbiol. 2005. PMID: 16333081 Free PMC article.
-
The measurement of HIV-1 viral load in resource-limited settings: how and where?Clin Lab. 2007;53(3-4):135-48. Clin Lab. 2007. PMID: 17447649 Review.
Cited by
-
An observational cohort comparison of facilitators of retention in care and adherence to anti-eetroviral therapy at an HIV treatment center in Kenya.PLoS One. 2012;7(3):e32727. doi: 10.1371/journal.pone.0032727. Epub 2012 Mar 9. PLoS One. 2012. PMID: 22427869 Free PMC article.
-
Performance characteristics of the Cavidi ExaVir viral load assay and the ultra-sensitive P24 assay relative to the Roche Monitor HIV-1 RNA assay.J Clin Virol. 2010 Nov;49(3):198-204. doi: 10.1016/j.jcv.2010.07.022. Epub 2010 Sep 15. J Clin Virol. 2010. PMID: 20832356 Free PMC article.
-
Comparative evaluation of the ExaVir Load version 3 reverse transcriptase assay for measurement of human immunodeficiency virus type 1 plasma load.J Clin Microbiol. 2009 Oct;47(10):3266-70. doi: 10.1128/JCM.00715-09. Epub 2009 Aug 5. J Clin Microbiol. 2009. PMID: 19656978 Free PMC article.
-
Can HIV reverse transcriptase activity assay be a low-cost alternative for viral load monitoring in resource-limited settings?BMJ Open. 2016 Jan 27;6(1):e008795. doi: 10.1136/bmjopen-2015-008795. BMJ Open. 2016. PMID: 26817634 Free PMC article. Clinical Trial.
-
Paper microchip with a graphene-modified silver nano-composite electrode for electrical sensing of microbial pathogens.Nanoscale. 2017 Feb 2;9(5):1852-1861. doi: 10.1039/c6nr06417e. Nanoscale. 2017. PMID: 27845796 Free PMC article.
References
-
- Awad, R. J., G. E. Corrigan, D. H. Ekstrand, R. Thorstensson, C. Kallander, and J. S. Gronowitz. 1997. Measurement of levels of human immunodeficiency virus type 1 reverse transcriptase (RT) and RT-activity-blocking antibody in humans by a new standardized colorimetric assay. J. Clin. Microbiol. 35:1080-1089. - PMC - PubMed
-
- Boni, J., M. Opravil, Z. Tomasik, M. Rothen, K. Bisset, P. Grob, R. Lüthy, and J. Schüpbach. 1997. Simple monitoring of antiretroviral therapy with a signal-amplification-boosted HIV p24 antigen assay with heat-denatured plasma. AIDS 11:F47-F52. - PubMed
-
- Braun, J., J. C. Plantier, M. F. Hellot, E. Tuaillon, M. Gueudin, F. Damond, A. Malmsten, G. E. Corrigan, and F. Simon. 2003. A new quantitative HIV viral load assay based on plasma virion reverse transcriptase activity for the different types, groups and subtypes. AIDS 17:331-336. - PubMed
-
- Brown, P. 2000. Cheaper AIDS drugs due for Third World. Nature 405:263. - PubMed
-
- Burgisser, P., P. Vernazza, M. Flepp, J. Boni, Z. Tomasik, U. Hummel, G. Pantaleo, and J. Schupbach. 2000. Performance of five different assays for the quantification of viral load in persons infected with various subtypes of HIV-1. Swiss HIV cohort study. J. Acquir. Immune Defic. Syndr. 23:138-144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

