Rap1b is required for normal platelet function and hemostasis in mice
- PMID: 15696195
- PMCID: PMC546455
- DOI: 10.1172/JCI22973
Rap1b is required for normal platelet function and hemostasis in mice
Erratum in
- J Clin Invest. 2005 Aug;115(8):2296
Abstract
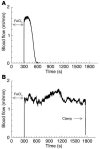
Rap1b, an abundant small GTPase in platelets, becomes rapidly activated upon stimulation with agonists. Though it has been implicated to act downstream from G protein-coupled receptors (GPCRs) and upstream of integrin alpha IIbbeta3, the precise role of Rap1b in platelet function has been elusive. Here we report the generation of a murine rap1b knockout and show that Rap1b deficiency results in a bleeding defect due to defective platelet function. Aggregation of Rap1b-null platelets is reduced in response to stimulation with both GPCR-linked and GPCR-independent agonists. Underlying the defective Rap1b-null platelet function is decreased activation of integrin alphaIIbbeta3 in response to stimulation with agonists and signaling downstream from the integrin alpha IIbbeta3. In vivo, Rap1b-null mice are protected from arterial thrombosis. These data provide genetic evidence that Rap1b is involved in a common pathway of integrin activation, is required for normal hemostasis in vivo, and may be a clinically relevant antithrombotic therapy target.
Figures




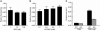
References
-
- Robbins S, Khosla M, Thiery R, Weeks G, Spiegelman GB. Ras-related genes in Dictyostelium discoideum. Dev. Genet. 1991;12:147–153. - PubMed
-
- Hariharan IK, Carthew RW, Rubin GM. The Drosophila roughened mutation: activation of a rap homolog disrupts eye development and interferes with cell determination. Cell. 1991;67:717–722. - PubMed
-
- Kang R, Kae H, Ip H, Spiegelman GB, Weeks G. Evidence for a role for the Dictyostelium Rap1 in cell viability and the response to osmotic stress. J. Cell Sci. 2002;115:3675–3682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

