An automated system for measuring parameters of nematode sinusoidal movement
- PMID: 15698479
- PMCID: PMC549551
- DOI: 10.1186/1471-2156-6-5
An automated system for measuring parameters of nematode sinusoidal movement
Abstract
Background: Nematode sinusoidal movement has been used as a phenotype in many studies of C. elegans development, behavior and physiology. A thorough understanding of the ways in which genes control these aspects of biology depends, in part, on the accuracy of phenotypic analysis. While worms that move poorly are relatively easy to describe, description of hyperactive movement and movement modulation presents more of a challenge. An enhanced capability to analyze all the complexities of nematode movement will thus help our understanding of how genes control behavior.
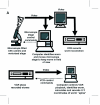
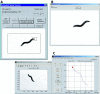
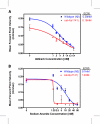
Results: We have developed a user-friendly system to analyze nematode movement in an automated and quantitative manner. In this system nematodes are automatically recognized and a computer-controlled microscope stage ensures that the nematode is kept within the camera field of view while video images from the camera are stored on videotape. In a second step, the images from the videotapes are processed to recognize the worm and to extract its changing position and posture over time. From this information, a variety of movement parameters are calculated. These parameters include the velocity of the worm's centroid, the velocity of the worm along its track, the extent and frequency of body bending, the amplitude and wavelength of the sinusoidal movement, and the propagation of the contraction wave along the body. The length of the worm is also determined and used to normalize the amplitude and wavelength measurements. To demonstrate the utility of this system, we report here a comparison of movement parameters for a small set of mutants affecting the Go/Gq mediated signaling network that controls acetylcholine release at the neuromuscular junction. The system allows comparison of distinct genotypes that affect movement similarly (activation of Gq-alpha versus loss of Go-alpha function), as well as of different mutant alleles at a single locus (null and dominant negative alleles of the goa-1 gene, which encodes Go-alpha). We also demonstrate the use of this system for analyzing the effects of toxic agents. Concentration-response curves for the toxicants arsenite and aldicarb, both of which affect motility, were determined for wild-type and several mutant strains, identifying P-glycoprotein mutants as not significantly more sensitive to either compound, while cat-4 mutants are more sensitive to arsenite but not aldicarb.
Conclusions: Automated analysis of nematode movement facilitates a broad spectrum of experiments. Detailed genetic analysis of multiple alleles and of distinct genes in a regulatory network is now possible. These studies will facilitate quantitative modeling of C. elegans movement, as well as a comparison of gene function. Concentration-response curves will allow rigorous analysis of toxic agents as well as of pharmacological agents. This type of system thus represents a powerful analytical tool that can be readily coupled with the molecular genetics of nematodes.
Figures





References
-
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode C. elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

