Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes
- PMID: 15698615
- PMCID: PMC2813490
- DOI: 10.1016/j.expneurol.2004.05.038
Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes
Abstract
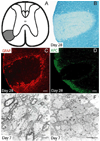
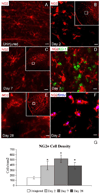
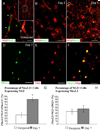
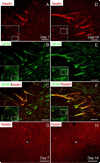
Chronic demyelination is a pathophysiologic component of compressive spinal cord injury (SCI) and a characteristic finding in demyelinating diseases including multiple sclerosis (MS). A better characterization of endogenous cells responsible for successful remyelination is essential for designing therapeutic strategies aimed at restoring functional myelin. The present study examined the spatiotemporal response of endogenous oligodendrocyte precursor cells (OPCs) following ethidium bromide (EB)-induced demyelination of the adult rat spinal cord. Beginning at 2 days post-EB injection (dpi), a robust mobilization of highly proliferative NG2(+) cells within the lesion was observed, none of which expressed the oligodendrocyte lineage-associated transcription factor Nkx2.2. At 7 dpi, a significant up-regulation of Nkx2.2 by OPCs within the lesion was observed, 90% of which coexpressed NG2 and virtually all of which coexpressed the bHLH transcription factor Olig2. Despite successful recruitment of Nkx2.2(+)/Olig2(+) OPCs within the lesion, demyelinated axons were not remyelinated by these OPCs in regions lacking astrocytes. Rather, Schwann cell remyelination predominated throughout the central core of the lesion, particularly around blood vessels. Oligodendrocyte remyelination was observed in the astrogliotic perimeter, suggesting a necessary role for astrocytes in oligodendrocyte maturation. In addition, reexpression of the radial glial antigen, RC-1, by reactive astrocytes and ependymal cells was observed following injury. However, these cells did not express the neural stem cell (NSC)-associated transcription factors Sox1 or Sox2, suggesting that the endogenous response is primarily mediated by glial progenitors. In vivo electrophysiology demonstrated a limited and unsustained functional recovery concurrent with endogenous remyelination following EB-induced lesions.
Figures
Similar articles
-
Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury.J Neurosci. 2010 Feb 24;30(8):2989-3001. doi: 10.1523/JNEUROSCI.3174-09.2010. J Neurosci. 2010. PMID: 20181596 Free PMC article.
-
The EIIIA domain from astrocyte-derived fibronectin mediates proliferation of oligodendrocyte progenitor cells following CNS demyelination.Glia. 2015 Feb;63(2):242-56. doi: 10.1002/glia.22748. Epub 2014 Aug 25. Glia. 2015. PMID: 25156142 Free PMC article.
-
Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS.Mol Cell Neurosci. 2004 Nov;27(3):247-54. doi: 10.1016/j.mcn.2004.06.015. Mol Cell Neurosci. 2004. PMID: 15519240
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
-
The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury.Glia. 2020 Feb;68(2):227-245. doi: 10.1002/glia.23706. Epub 2019 Aug 21. Glia. 2020. PMID: 31433109 Review.
Cited by
-
Human iPSC-Derived Immature Astroglia Promote Oligodendrogenesis by Increasing TIMP-1 Secretion.Cell Rep. 2016 May 10;15(6):1303-15. doi: 10.1016/j.celrep.2016.04.011. Epub 2016 Apr 28. Cell Rep. 2016. PMID: 27134175 Free PMC article.
-
Developmental and injury-induced expression of alpha1beta1 and alpha6beta1 integrins in the rat spinal cord.Brain Res. 2007 Jan 26;1130(1):54-66. doi: 10.1016/j.brainres.2006.10.072. Epub 2006 Dec 11. Brain Res. 2007. PMID: 17161391 Free PMC article.
-
Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging.Biomolecules. 2021 Dec 15;11(12):1887. doi: 10.3390/biom11121887. Biomolecules. 2021. PMID: 34944531 Free PMC article. Review.
-
Inhibitors of myelination: ECM changes, CSPGs and PTPs.Exp Neurol. 2014 Jan;251:39-46. doi: 10.1016/j.expneurol.2013.10.017. Epub 2013 Nov 4. Exp Neurol. 2014. PMID: 24200549 Free PMC article.
-
Nogo receptor inhibition enhances functional recovery following lysolecithin-induced demyelination in mouse optic chiasm.PLoS One. 2014 Sep 3;9(9):e106378. doi: 10.1371/journal.pone.0106378. eCollection 2014. PLoS One. 2014. PMID: 25184636 Free PMC article.
References
-
- Aubert J, Stavridis MP, Tweedie S, O’Reilly M, Vierlinger K, Li M, Ghazal P, Pratt T, Mason JO, Roy D, Smith A. Screening for mammalian neural genes via fluorescence-activated cell sorter purification of neural precursors from Sox1-gfp knock-in mice. Proc. Natl. Acad. Sci. U. S. A. 2003;100 Suppl. 1:11836–11841. - PMC - PubMed
-
- Berry M, Hubbard P, Butt AM. Cytology and lineage of NG2-positive glia. J. Neurocytol. 2002;31:457–467. - PubMed
-
- Blakemore WF. Ethidium bromide induced demyelination in the spinal cord of the cat. Neuropathol. Appl. Neurobiol. 1982;8:365–375. - PubMed
-
- Blakemore WF. Transplanted cultured type-1 astrocytes can be used to reconstitute the glia limitans of the CNS: the structure which prevents Schwann cells from myelinating CNS axons. Neuropathol. Appl. Neurobiol. 1992;18:460–466. - PubMed
-
- Blakemore WF, Crang AJ. Extensive oligodendrocyte remyelination following injection of cultured central nervous system cells into demyelinating lesions in adult central nervous system. Dev. Neurosci. 1988;10:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

