Synthesis of 4-hydroxy-1-methylindole and benzo[b]thiophen-4-ol based unnatural flavonoids as new class of antimicrobial agents
- PMID: 15698765
- PMCID: PMC7125744
- DOI: 10.1016/j.bmc.2004.12.032
Synthesis of 4-hydroxy-1-methylindole and benzo[b]thiophen-4-ol based unnatural flavonoids as new class of antimicrobial agents
Abstract
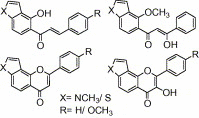
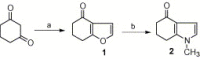
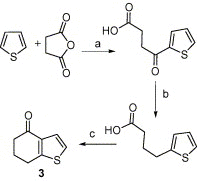
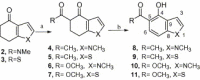
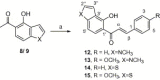
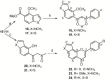
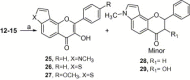
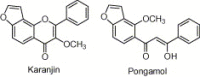
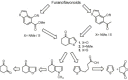
Synthesis of nitrogen and sulfur heterocyclic mimics of furanoflavonoids have been achieved for the first time. Synthesized flavonoid alkaloids and thiophenyl flavonoids have been screened for antifungal and antibacterial activities. All the test compounds barring 25 exhibited antifungal activity. The compound 19 was the best and showed comparable MICs to the known compound karanjin. Compounds 5, 12, 14 and 22 also showed comparable MIC to karanjin.
Figures
Similar articles
-
Synthesis and antimicrobial activity of some new flavonyl oxime ether derivatives.Arzneimittelforschung. 1999 Oct;49(10):853-7. doi: 10.1055/s-0031-1300514. Arzneimittelforschung. 1999. PMID: 10554664
-
Design and synthesis of novel antimicrobial acyclic and heterocyclic dyes and their precursors for dyeing and/or textile finishing based on 2-N-acylamino-4,5,6,7-tetrahydro-benzo[b]thiophene systems.Molecules. 2011 Jul 26;16(8):6271-305. doi: 10.3390/molecules16086271. Molecules. 2011. PMID: 21792147 Free PMC article.
-
Synthesis and antimicrobial activity of flavone-3'-carboxaldehyde oxime ether derivatives.Arzneimittelforschung. 2003;53(7):522-5. doi: 10.1055/s-0031-1297143. Arzneimittelforschung. 2003. PMID: 12918219
-
Synthesis of heterocyclic compounds through palladium-catalyzed C-H cyclization processes.Chem Pharm Bull (Tokyo). 2013;61(10):987-96. doi: 10.1248/cpb.c13-00420. Chem Pharm Bull (Tokyo). 2013. PMID: 24088691 Review.
-
Recent Developments in the Synthesis and Antimicrobial Activity of Indole and its Derivatives.Curr Org Synth. 2019;16(1):17-37. doi: 10.2174/1570179415666181113144939. Curr Org Synth. 2019. PMID: 31965921 Review.
Cited by
-
4,5,6,7-Tetrahydroindol-4-Ones as a Valuable Starting Point for the Synthesis of Polyheterocyclic Structures.Molecules. 2021 Jul 29;26(15):4596. doi: 10.3390/molecules26154596. Molecules. 2021. PMID: 34361747 Free PMC article. Review.
-
The design, synthesis and biological evaluation of conformationally restricted 4-substituted-2,6-dimethylfuro[2,3-d]pyrimidines as multi-targeted receptor tyrosine kinase and microtubule inhibitors as potential antitumor agents.Bioorg Med Chem. 2015 May 15;23(10):2408-23. doi: 10.1016/j.bmc.2015.03.061. Epub 2015 Mar 30. Bioorg Med Chem. 2015. PMID: 25882519 Free PMC article.
-
Novel indazolylchromones: synthesis, fungicidal evaluation, molecular docking and aquatic toxicity prediction.Front Chem. 2024 Jun 11;12:1411187. doi: 10.3389/fchem.2024.1411187. eCollection 2024. Front Chem. 2024. PMID: 38919273 Free PMC article.
-
Design, synthesis, biological evaluation and X-ray crystal structure of novel classical 6,5,6-tricyclic benzo[4,5]thieno[2,3-d]pyrimidines as dual thymidylate synthase and dihydrofolate reductase inhibitors.Bioorg Med Chem. 2011 Jun 1;19(11):3585-94. doi: 10.1016/j.bmc.2011.03.067. Epub 2011 Apr 9. Bioorg Med Chem. 2011. PMID: 21550809 Free PMC article.
-
Synthesis and Biological Activity of 2',3'-iso-Aryl-abscisic Acid Analogs.Molecules. 2017 Dec 15;22(12):2229. doi: 10.3390/molecules22122229. Molecules. 2017. PMID: 29244719 Free PMC article.
References
-
- Clark T.A., Hajjeh R.A. Curr. Opin. Infect. Dis. 2002;15:569–574. - PubMed
-
- Hage C.A., Goldman M., Wheat L.J. Eur. J. Med. Res. 2002;7:236–241. - PubMed
-
- Wang H., Ding Y., Li X., Yang L., Zhang W., Kang W. N. Engl. J. Med. 2003;349:07–508. - PubMed
-
- Dixon D.M. J. Appl. Microbiol. 2001;91:602–605. - PubMed
-
- Romani L. Nat. Rev. Immunol. 2004;4:11–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

