CCR8 is expressed by antigen-elicited, IL-10-producing CD4+CD25+ T cells, which regulate Th2-mediated granuloma formation in mice
- PMID: 15699124
- PMCID: PMC1599789
- DOI: 10.4049/jimmunol.174.4.1962
CCR8 is expressed by antigen-elicited, IL-10-producing CD4+CD25+ T cells, which regulate Th2-mediated granuloma formation in mice
Abstract
CCR8 was initially described as a Th2 cell-restricted receptor, but this has not been fully tested in vivo. The present study used ex vivo and in vivo approaches to examine the distribution and functional significance of CCR8 among CD4+ T cells. Populations of cytokine-secreting CD4+ T cells were generated in primed mice with Th1 or Th2 cell-mediated pulmonary granulomas, respectively elicited by i.v. challenge with either Mycobacteria bovis purified protein derivative- or Schistosoma mansoni egg Ag (SEA)-coated beads. Cytokine-producing CD4+ T cells were isolated from Ag-stimulated draining lymph node cultures by positive selection. Quantitative analysis of cytokine mRNA indicated enriched populations of IFN-gamma-, IL-4-, and IL-10-producing cells. Analysis of chemokine receptor mRNA indicated that IL-10+ cells selectively expressed CCR8 in the SEA bead-elicited type 2 response. The IL-10+CCR8+ populations were CD25+ and CD44+ but lacked enhanced Foxp3 expression. Adoptive transfer to naive recipients indicated that IL-10+ T cells alone could not transfer type 2 inflammation. Analysis of SEA bead-challenged CCR8-/- mice indicated significantly impaired IL-10 production as well as reductions in granuloma eosinophils. Adoptive transfer of CD4+CCR8+/+ T cells corrected cytokine and inflammation defects, but the granuloma eosinophil recruitment defect persisted when donor cells were depleted of IL-10+ cells. Accordingly, local IL-10 production correlated with CCR8 ligand (CCL1) expression and the appearance of CCR8+ cells in granulomatous lungs. Thus, IL-10-producing, CCR8+CD4+CD25+CD44+ T cells are generated during SEA challenge, which augment the Th2-mediated eosinophil-rich response to the parasite Ags.
Figures


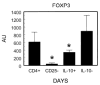
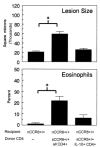



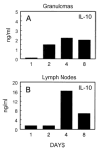


Similar articles
-
CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes.J Immunol. 2006 Nov 15;177(10):6940-51. doi: 10.4049/jimmunol.177.10.6940. J Immunol. 2006. PMID: 17082609
-
Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor.Am J Pathol. 1994 Nov;145(5):1105-13. Am J Pathol. 1994. PMID: 7977642 Free PMC article.
-
Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-gamma and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks.J Immunol. 1997 Oct 1;159(7):3565-73. J Immunol. 1997. PMID: 9317156
-
Alloantigen specific T regulatory cells in transplant tolerance.Int Immunopharmacol. 2009 May;9(5):570-4. doi: 10.1016/j.intimp.2009.01.016. Epub 2009 Jan 29. Int Immunopharmacol. 2009. PMID: 19539571 Review.
-
The debate over the effector function of eosinophils in helminth infection: new evidence from studies on the regulation of vaccine immunity by IL-12.Mem Inst Oswaldo Cruz. 1997;92 Suppl 2:105-8. doi: 10.1590/s0074-02761997000800014. Mem Inst Oswaldo Cruz. 1997. PMID: 9698921 Review.
Cited by
-
A key role for CC chemokine receptor 1 in T-cell-mediated respiratory inflammation.Am J Pathol. 2008 Feb;172(2):386-94. doi: 10.2353/ajpath.2008.070537. Epub 2008 Jan 17. Am J Pathol. 2008. PMID: 18202190 Free PMC article.
-
Acquisition of suppressive function by conventional T cells limits antitumor immunity upon Treg depletion.Sci Immunol. 2023 Dec 15;8(90):eabo5558. doi: 10.1126/sciimmunol.abo5558. Epub 2023 Dec 15. Sci Immunol. 2023. PMID: 38100544 Free PMC article.
-
Immunopathology of schistosomiasis.Immunol Cell Biol. 2007 Feb-Mar;85(2):148-54. doi: 10.1038/sj.icb.7100014. Epub 2006 Dec 12. Immunol Cell Biol. 2007. PMID: 17160074 Free PMC article. Review.
-
IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni.PLoS Pathog. 2011 Aug;7(8):e1002171. doi: 10.1371/journal.ppat.1002171. Epub 2011 Aug 4. PLoS Pathog. 2011. PMID: 21829367 Free PMC article.
-
CD4 regulatory T cells in human cancer pathogenesis.Cancer Immunol Immunother. 2007 Mar;56(3):271-85. doi: 10.1007/s00262-006-0194-y. Epub 2006 Jul 4. Cancer Immunol Immunother. 2007. PMID: 16819631 Free PMC article. Review.
References
-
- Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129. - PubMed
-
- Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344. - PubMed
-
- Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

