Alpha-helix formation in a photoswitchable peptide tracked from picoseconds to microseconds by time-resolved IR spectroscopy
- PMID: 15699340
- PMCID: PMC548979
- DOI: 10.1073/pnas.0406948102
Alpha-helix formation in a photoswitchable peptide tracked from picoseconds to microseconds by time-resolved IR spectroscopy
Abstract
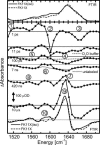
Photo-triggered alpha-helix formation of a 16-residue peptide featuring a built-in conformational photoswitch is monitored by time-resolved IR spectroscopy. An experimental approach with 2-ps time resolution and a scanning range up to 30 micros is used to cover all time scales of the peptide dynamics. Experiments are carried out at different temperatures between 281 and 322 K. We observe single-exponential kinetics of the amide I' band at 322 K on a time scale comparable to a recent temperature-jump folding experiment. When lowering the temperature, the kinetics become slower and nonexponential. The transition is strongly activated. Spectrally dispersed IR measurements provide multiple spectroscopic probes simultaneously in one experiment by resolving the amide I' band, isotope-labeled amino acid residues, and side chains. We find differing relaxation dynamics at different spectral positions.
Figures




References
-
- Daniel, R. M., Dunn, R. V., Finney, J. L. & Smith, J. C. (2003) Annu. Rev. Biophys. Biomol. Struct. 32, 69-92. - PubMed
-
- Karplus, M. (2000) J. Phys. Chem. B 104, 11-27.
-
- Gruebele, M. (2002) Curr. Opin. Struct. Biol. 12, 161-168. - PubMed
-
- Ferguson, N. & Fersht, A. R. (2003) Curr. Opin. Struct. Biol. 13, 75-81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

