Quantitative PCR-enhanced immunoassay for measurement of enteroviral immunoglobulin M antibody and diagnosis of aseptic meningitis
- PMID: 15699416
- PMCID: PMC549296
- DOI: 10.1128/CDLI.12.2.235-241.2005
Quantitative PCR-enhanced immunoassay for measurement of enteroviral immunoglobulin M antibody and diagnosis of aseptic meningitis
Abstract
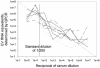
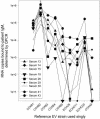
A PCR-enhanced immunoassay (PIA) to detect enterovirus (EV) immunoglobulin M (IgM) for diagnosis of recent EV infection was recently developed. This test was compared with another EV IgM capture technique, the solid-phase reverse immunosorbent test (SPRIST). Fourteen of 43 serum samples from aseptic meningitis patients were positive by PIA, whereas 10 were positive by SPRIST. One of 39 control serum samples was weakly positive by PIA. A single-serum-dilution real-time PCR-based PIA for EV IgM (quantitative PIA [QPIA]) was also developed and evaluated against PIA, SPRIST, an EV IgM radioimmunoassay (RIA), and clinical data. A mixture of 12 EVs was used as the antigen. Results from investigating four groups of serum samples were as follows. (i) The nine PIA-positive serum samples in group 1 were all positive by QPIA. (ii) Group 2 consisted of 59 serum samples from aseptic meningitis patients. Nineteen of 30 serum samples (63%) taken at hospital admission were positive by QPIA. Of these, 17 were positive in EV PCR. (iii) None of the 30 control serum samples in group 3 were positive by QPIA. (iv) For the 24 serum samples in group 4, of which 11 were positive and 13 were negative by RIA, the QPIA results were completely concordant. The sensitivity and specificity of QPIA for diagnosis of EV infection were 70 and 80%, respectively. QPIA provides a rational strategy for the detection of EV IgM, allows the use of viral antigens with minimal purification, and needs no virus-specific reagents apart from those in the PCR. QPIA is a generally applicable method for the detection of viral IgM in IgM capture assays.
Figures

Similar articles
-
Early diagnosis of enteroviral meningitis by detection of specific IgM antibodies with a solid-phase reverse immunosorbent test (SPRIST) and mu-capture EIA.J Med Virol. 1992 Mar;36(3):193-201. doi: 10.1002/jmv.1890360309. J Med Virol. 1992. PMID: 1314285
-
Recent enterovirus infection in type 1 diabetes: evidence with a novel IgM method.J Med Virol. 2007 Dec;79(12):1861-7. doi: 10.1002/jmv.21008. J Med Virol. 2007. PMID: 17935175
-
[Rational and efficient enterovirus diagnosis].Dtsch Med Wochenschr. 2001 Mar 16;126(11):289-93. doi: 10.1055/s-2001-11880. Dtsch Med Wochenschr. 2001. PMID: 11296567 German.
-
Aseptic meningitis.Curr Opin Infect Dis. 2007 Jun;20(3):272-7. doi: 10.1097/QCO.0b013e3280ad4672. Curr Opin Infect Dis. 2007. PMID: 17471037 Review.
-
The child with aseptic meningitis.Pediatr Case Rev. 2003 Oct;3(4):218-21. doi: 10.1097/01.PCA.0000083250.03358.9F. Pediatr Case Rev. 2003. PMID: 14520085 Review. No abstract available.
Cited by
-
Acute viral infections of the central nervous system in immunocompetent adults: diagnosis and management.Drugs. 2013 Feb;73(2):131-58. doi: 10.1007/s40265-013-0007-5. Drugs. 2013. PMID: 23377760 Review.
-
The modern autopsy: what to do if infection is suspected.Arch Med Res. 2005 Nov-Dec;36(6):713-23. doi: 10.1016/j.arcmed.2005.04.006. Arch Med Res. 2005. PMID: 16216653 Free PMC article. Review.
-
Airborne transmission of SARS-CoV-2: The world should face the reality.Environ Int. 2020 Jun;139:105730. doi: 10.1016/j.envint.2020.105730. Epub 2020 Apr 10. Environ Int. 2020. PMID: 32294574 Free PMC article.
-
Enteroviruses as a possible cause of hypertension, dilated cardiomyopathy (DCM) and hypertensive heart failure (HHF) in South western Nigeria.Afr Health Sci. 2013 Dec;13(4):1098-106. doi: 10.4314/ahs.v13i4.34. Afr Health Sci. 2013. PMID: 24940338 Free PMC article.
References
-
- Aspholm, R., S. Zuo, J. Fohlman, G. Frisk, G. Friman, and J. Blomberg. 1999. A novel serological technique: polymerase chain reaction enhanced immunoassay. Application to enterovirus IgM diagnosis. J. Virol. Methods 80:187-196. - PubMed
-
- Bendig, J. W., and P. Molyneaux. 1996. Sensitivity and specificity of mu-capture ELISA for detection of enterovirus IgM. J. Virol. Methods 59:23-32. - PubMed
-
- Casas, I., P. E. Klapper, G. M. Cleator, J. E. Echevarria, A. Tenorio, and J. M. Echevarria. 1995. Two different PCR assays to detect enteroviral RNA in CSF samples from patients with acute aseptic meningitis. J. Med. Virol. 47:378-385. - PubMed
-
- Chang, L. Y., T. Y. Lin, K. H. Hsu, Y. C. Huang, K. L. Lin, C. Hsueh, S. R. Shih, H. C. Ning, M. S. Hwang, H. S. Wang, and C. Y. Lee. 1999. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 354:1682-1686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

