Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism
- PMID: 15700028
- PMCID: PMC1576089
- DOI: 10.1038/sj.bjp.0706134
Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism
Abstract
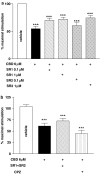
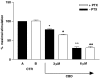
We evaluated the ability of cannabidiol (CBD) to impair the migration of tumor cells stimulated by conditioned medium. CBD caused concentration-dependent inhibition of the migration of U87 glioma cells, quantified in a Boyden chamber. Since these cells express both cannabinoid CB1 and CB2 receptors in the membrane, we also evaluated their engagement in the antimigratory effect of CBD. The inhibition of cell was not antagonized either by the selective cannabinoid receptor antagonists SR141716 (CB1) and SR144528 (CB2) or by pretreatment with pertussis toxin, indicating no involvement of classical cannabinoid receptors and/or receptors coupled to Gi/o proteins. These results reinforce the evidence of antitumoral properties of CBD, demonstrating its ability to limit tumor invasion, although the mechanism of its pharmacological effects remains to be clarified.
Figures


References
-
- BISOGNO T., HANUS L., DE PETROCELLIS L., TCHILIBON S., PONDE D.E., BRANDI I., MORIELLO A.S., DAVIS J.B., MECHOULAM R., DI MARZO V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134:845–852. - PMC - PubMed
-
- CARLISLE S.J., MARCIANO-CABRAL F., STAAB A., LUDWICK C., CABRAL G.A. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2002;2:69–82. - PubMed
-
- GUZMAN M. Cannabinoids: potential anticancer agents. Nat. Rev. Cancer. 2003;3:745–755. - PubMed
-
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. - PubMed
-
- JARAI Z., WAGNER J.A., VARGA K., LAKE K.D., COMPTON D.R., MARTIN B.R., ZIMMER A.M., BONNER T.I., BUCKLEY N.E., MEZEY E., RAZDAN R.K., ZIMMER A., KUNOS G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14136–14141. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

