The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles
- PMID: 15701693
- PMCID: PMC548330
- DOI: 10.1073/pnas.0409781102
The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles
Abstract
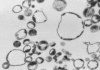
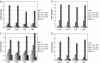
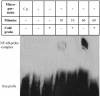
Rheumatoid arthritis is a chronic inflammatory disease characterized by destruction of cartilage and bone that is mediated by synovial fibroblasts. To determine the mechanisms by which these cells are activated to produce matrix metalloproteinases (MMPs), the effects of microparticles were investigated. Microparticles are small membrane-bound vesicles whose release from immune cells is increased during activation and apoptosis. Because microparticles occur abundantly in the synovial fluid in rheumatoid arthritis, they could represent novel stimulatory agents. Microparticles derived from T cells and monocytes strongly induced the synthesis of MMP-1, MMP-3, MMP-9, and MMP-13 in fibroblasts. The induction was time-dependent, with effects primarily observed after 36 h; under these conditions, MMP-2, MMP-14, and tissue inhibitor of MMP-1 (TIMP-1), TIMP-2, and TIMP-3 were not induced. Microparticles also increased the synthesis of inflammatory mediators including IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), and MCP-2. In Ikappa-B-transfected synovial fibroblasts, MMPs were less inducible by microparticles compared with wild-type fibroblasts. Blocking of TNFalpha and IL-1beta with antibodies against TNFalpha and with IL-1 receptor antagonist did not abrogate stimulation by microparticles. These data provide evidence for a novel mechanism by which vesicles derived from activated or apoptotic immune cells can promote the destructive activity of synovial fibroblasts in rheumatoid arthritis.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

