Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane
- PMID: 15701694
- PMCID: PMC549020
- DOI: 10.1073/pnas.0409741102
Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane
Abstract
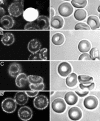
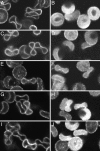
To characterize the location of glycolytic enzymes (GEs) in intact human erythrocytes, freshly drawn blood was fixed and stained with Abs to GAPDH, aldolase, phosphofructokinase (PFK), pyruvate kinase (PK), lactate dehydrogenase (LDH), carbonic anhydrase II, Hb, and band 3 (AE1). Confocal microscopy revealed that in cells where band 3 displays its expected membrane staining and Hb is evenly distributed across the cytoplasm, GEs are largely limited to the membrane. Biochemical studies confirmed that the membrane binding sites for GAPDH, aldolase, and PFK reside on band 3, but related analyses demonstrate that sites for PK and LDH do not. Four lines of evidence demonstrate that the GEs are at least partially assembled into multimeric complexes near the NH2 terminus of band 3. First, a mAb to residues 1-12 of band 3 displaces all of the above GEs from the membrane, including LDH and PK, which do not bind band 3. Second, tyrosine phosphorylation of the NH2 terminus of band 3 (Y8 and Y21) reversibly releases all of the GEs from the membrane, including LDH and PK. Third, deoxygenation of RBCs dislodges all GEs from the membrane, consistent with the established ability of deoxyHb but not oxyHb to bind the NH2 terminus of band 3. Fourth, a large increase in the accessibility of enzyme epitopes is observed upon dissociation of GEs from the membrane. We conclude, therefore, that GEs are organized into complexes on the membrane whose assembly is regulated by oxygenation and phosphorylation.
Figures



References
-
- Schrier, S. L. (1966) Am. J. Physiol. 210, 139-145. - PubMed
-
- Tsai, I. H., Murthy, S. N. & Steck, T. L. (1982) J. Biol. Chem. 257, 1438-1442. - PubMed
-
- Murthy, S. N., Liu, T., Kaul, R. K., Kohler, H. & Steck, T. L. (1981) J. Biol. Chem. 256, 11203-11208. - PubMed
-
- Higashi, T., Richards, C. S. & Uyeda, K. (1979) J. Biol. Chem. 54, 9542-9550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

