The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin
- PMID: 15701696
- PMCID: PMC549477
- DOI: 10.1073/pnas.0409719102
The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin
Abstract
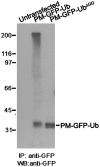
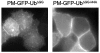
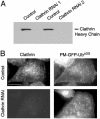
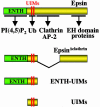
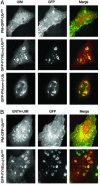
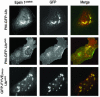
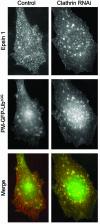
Monoubiquitination of plasma membrane proteins is a mechanism to control their endocytic trafficking by promoting their interaction with cytosolic adaptor proteins that contain ubiquitin (Ub)-binding domains. Epsin, which contains Ub interaction motifs (UIMs), as well as binding sites for the clathrin coat and clathrin accessory factors, is thought to function as one of such adaptors. The importance of clathrin in the internalization of ubiquitinated cargo, however, has been questioned. Here, we show that a GFP-Ub chimera directly targeted to the plasma membrane via a lipid-based interaction is efficiently taken up by endocytosis and delivered to the same endosomes that accumulate internalized EGF. Internalization of the chimera requires integrity of the UIM binding interface of Ub, but does not require clathrin. Surprisingly, WT epsin showed little colocalization with this chimera, whereas UIM-containing epsin constructs that lack the clathrin and AP2 binding region, strikingly colocalized with this chimera on endocytic vacuoles. In addition, extensive colocalization of WT epsin with the chimera on endocytic structures could be observed in cells where clathrin levels were drastically reduced by RNA interference. Our results reveal an important regulatory mechanism in epsin function. The mutually exclusive colocalization of epsin with membrane-bound Ub or clathrin may play a role in controlling the endocytic route taken by ubiquitinated cargo.
Figures


Comment in
-
Endocytosis of membrane receptors: two pathways are better than one.Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):2679-80. doi: 10.1073/pnas.0500213102. Epub 2005 Feb 14. Proc Natl Acad Sci U S A. 2005. PMID: 15710869 Free PMC article. No abstract available.
References
-
- Hershko, A. & Ciechanover, A. (1998) Annu. Rev. Biochem. 67, 425–479. - PubMed
-
- Hicke, L. & Dunn, R. (2003) Annu. Rev. Cell. Dev. Biol. 19, 141–172. - PubMed
-
- Pickart, C. M. (2001) Annu. Rev. Biochem. 70, 503–533. - PubMed
-
- Aguilar R. C. & Wendland, B. (2003) Curr. Opin. Cell Biol. 15, 184–190. - PubMed
-
- Chen, H., Fre, S., Slepnev, V. I., Capua, M. R., Takei, K., Butler, M. H., Di Fiore, P. P. & De Camilli, P. (1998) Nature 394, 793–797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

