Overlapping roles of the cytoplasmic and mitochondrial redox regulatory systems in the yeast Saccharomyces cerevisiae
- PMID: 15701801
- PMCID: PMC549330
- DOI: 10.1128/EC.4.2.392-400.2005
Overlapping roles of the cytoplasmic and mitochondrial redox regulatory systems in the yeast Saccharomyces cerevisiae
Abstract
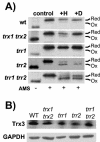
Thioredoxins are small, highly conserved oxidoreductases which are required to maintain the redox homeostasis of the cell. Saccharomyces cerevisiae contains a cytoplasmic thioredoxin system (TRX1, TRX2, and TRR1) as well as a complete mitochondrial thioredoxin system, comprising a thioredoxin (TRX3) and a thioredoxin reductase (TRR2). In the present study we have analyzed the functional overlap between the two systems. By constructing mutant strains with deletions of both the mitochondrial and cytoplasmic systems (trr1 trr2 and trx1 trx2 trx3), we show that cells can survive in the absence of both systems. Analysis of the redox state of the cytoplasmic thioredoxins reveals that they are maintained independently of the mitochondrial system. Similarly, analysis of the redox state of Trx3 reveals that it is maintained in the reduced form in wild-type cells and in mutants lacking components of the cytoplasmic thioredoxin system (trx1 trx2 or trr1). Surprisingly, the redox state of Trx3 is also unaffected by the loss of the mitochondrial thioredoxin reductase (trr2) and is largely maintained in the reduced form unless cells are exposed to an oxidative stress. Since glutathione reductase (Glr1) has been shown to colocalize to the cytoplasm and mitochondria, we examined whether loss of GLR1 influences the redox state of Trx3. During normal growth conditions, deletion of TRR2 and GLR1 was found to result in partial oxidation of Trx3, indicating that both Trr2 and Glr1 are required to maintain the redox state of Trx3. The oxidation of Trx3 in this double mutant is even more pronounced during oxidative stress or respiratory growth conditions. Taken together, these data indicate that Glr1 and Trr2 have an overlapping function in the mitochondria.
Figures







Similar articles
-
Oxidation of the yeast mitochondrial thioredoxin promotes cell death.Antioxid Redox Signal. 2013 Feb 1;18(4):376-85. doi: 10.1089/ars.2012.4597. Epub 2012 Aug 27. Antioxid Redox Signal. 2013. PMID: 22770501 Free PMC article.
-
Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems.EMBO Rep. 2003 Feb;4(2):184-8. doi: 10.1038/sj.embor.embor729. EMBO Rep. 2003. PMID: 12612609 Free PMC article.
-
Glutathione peroxidase 2 in Saccharomyces cerevisiae is distributed in mitochondria and involved in sporulation.Biochem Biophys Res Commun. 2011 Aug 5;411(3):580-5. doi: 10.1016/j.bbrc.2011.06.189. Epub 2011 Jul 6. Biochem Biophys Res Commun. 2011. PMID: 21763276
-
Thioredoxin and glutathione systems in Plasmodium falciparum.Int J Med Microbiol. 2012 Oct;302(4-5):187-94. doi: 10.1016/j.ijmm.2012.07.007. Epub 2012 Aug 29. Int J Med Microbiol. 2012. PMID: 22939033 Review.
-
The mitochondrial thioredoxin system.Antioxid Redox Signal. 2000 Winter;2(4):801-10. doi: 10.1089/ars.2000.2.4-801. Antioxid Redox Signal. 2000. PMID: 11213484 Review.
Cited by
-
The Incomplete Glutathione Puzzle: Just Guessing at Numbers and Figures?Antioxid Redox Signal. 2017 Nov 20;27(15):1130-1161. doi: 10.1089/ars.2017.7123. Epub 2017 Jul 19. Antioxid Redox Signal. 2017. PMID: 28540740 Free PMC article. Review.
-
Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state.EMBO J. 2012 Jun 15;31(14):3169-82. doi: 10.1038/emboj.2012.165. EMBO J. 2012. PMID: 22705944 Free PMC article.
-
Deciphering the Host-Pathogen Interactome of the Wheat-Common Bunt System: A Step towards Enhanced Resilience in Next Generation Wheat.Int J Mol Sci. 2022 Feb 26;23(5):2589. doi: 10.3390/ijms23052589. Int J Mol Sci. 2022. PMID: 35269732 Free PMC article.
-
Genetic and biochemical analysis of high iron toxicity in yeast: iron toxicity is due to the accumulation of cytosolic iron and occurs under both aerobic and anaerobic conditions.J Biol Chem. 2011 Feb 4;286(5):3851-62. doi: 10.1074/jbc.M110.190959. Epub 2010 Nov 29. J Biol Chem. 2011. PMID: 21115478 Free PMC article.
-
A Role for COX20 in Tolerance to Oxidative Stress and Programmed Cell Death in Saccharomyces cerevisiae.Microorganisms. 2019 Nov 18;7(11):575. doi: 10.3390/microorganisms7110575. Microorganisms. 2019. PMID: 31752220 Free PMC article.
References
-
- Boveris, A., and E. Cadenas. 1982. Production of superoxide radicals and hydrogen peroxide in mitochondria, p. 15-30. In L. W. Oberley (ed.), Superoxide dismutases, vol. 2. CRC Press, Boca Raton, Fla.
-
- Carmel-Harel, O., and G. Storz. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54:439-461. - PubMed
-
- Chance, B., H. Sies, and A. Boveris. 1979. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59:527-605. - PubMed
-
- Collinson, E. J., and C. M. Grant. 2003. Role of yeast glutaredoxins as glutathione S-transferases. J. Biol. Chem. 278:22492-22497. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

