Mispolarization of desmosomal proteins and altered intercellular adhesion in autosomal dominant polycystic kidney disease
- PMID: 15701820
- PMCID: PMC3432402
- DOI: 10.1152/ajprenal.00008.2005
Mispolarization of desmosomal proteins and altered intercellular adhesion in autosomal dominant polycystic kidney disease
Abstract
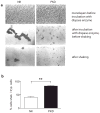
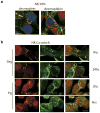
Polycystin-1, the product of the major gene mutated in autosomal dominant polycystic kidney disease (ADPKD), has been shown to associate with multiple epithelial cell junctions. Our hypothesis is that polycystin-1 is an important protein for the initial establishment of cell-cell junctions and maturation of the cell and that polycystin-1 localization is dependent on the degree of cell polarization. Using laser-scanning confocal microscopy and two models of cell polarization, polycystin-1 and desmosomes were found to colocalize during the initial establishment of cell-cell contact when junctions were forming. However, colocalization was lost in confluent monolayers. Parallel morphological and biochemical evaluations revealed a profound mispolarization of desmosomal components to both the apical and basolateral domains in primary ADPKD cells and tissue. Studies of the intermediate filament network associated with desmosomes showed that there is a decrease in cytokeratin levels and an abnormal expression of the mesenchymal protein vimentin in the disease. Moreover, we show for the first time that the structural alterations seen in adherens and desmosomal junctions have a functional impact, leaving the ADPKD cells with weakened cell-cell adhesion. In conclusion, in this paper we show that polycystin-1 transiently colocalizes with desmosomes and that desmosomal proteins are mislocalized as a consequence of polycystin-1 mutation.
Figures





References
-
- Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med. 2001;52:93–123. - PubMed
-
- Sutters M, Germino GG. Autosomal dominant polycystic kidney disease: molecular genetics and pathophysiology. J Lab Clin Med. 2003;141:91–101. - PubMed
-
- Grantham JJ, Geiser JL, Evan AP. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987;31:1145–1152. - PubMed
-
- Wilson PD. Polycystin: new aspects of structure, function, and regulation. J Am Soc Nephrol. 2001;12:834–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

