Developmental activation of the Rb-E2F pathway and establishment of cell cycle-regulated cyclin-dependent kinase activity during embryonic stem cell differentiation
- PMID: 15703208
- PMCID: PMC1073679
- DOI: 10.1091/mbc.e04-12-1056
Developmental activation of the Rb-E2F pathway and establishment of cell cycle-regulated cyclin-dependent kinase activity during embryonic stem cell differentiation
Abstract
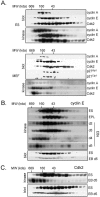
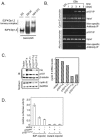
To understand cell cycle control mechanisms in early development and how they change during differentiation, we used embryonic stem cells to model embryonic events. Our results demonstrate that as pluripotent cells differentiate, the length of G(1) phase increases substantially. At the molecular level, this is associated with a significant change in the size of active cyclin-dependent kinase (Cdk) complexes, the establishment of cell cycle-regulated Cdk2 activity and the activation of a functional Rb-E2F pathway. The switch from constitutive to cell cycle-dependent Cdk2 activity coincides with temporal changes in cyclin A2 and E1 protein levels during the cell cycle. Transcriptional mechanisms underpin the down-regulation of cyclin levels and the establishment of their periodicity during differentiation. As pluripotent cells differentiate and pRb/p107 kinase activities become cell cycle dependent, the E2F-pRb pathway is activated and imposes cell cycle-regulated transcriptional control on E2F target genes, such as cyclin E1. These results suggest the existence of a feedback loop where Cdk2 controls its own activity through regulation of cyclin E1 transcription. Changes in rates of cell division, cell cycle structure and the establishment of cell cycle-regulated Cdk2 activity can therefore be explained by activation of the E2F-pRb pathway.
Figures



References
-
- Ashizawa, S., Nishizawa, H., Yamada, M., Higashi, H., Kondo, T., Ozawa, H., Kakita, A., and Hatakeyama, M. (2001). Collective inhibition of pRB family proteins by phosphorylation in cells with p16INK4a loss or cyclin E overexpression. J. Biol. Chem. 276, 11362-11370. - PubMed
-
- Breeden, L. L. (2003). Periodic transcription: a cycle within a cycle. Curr. Biol. 13, R31-R38. - PubMed
-
- Cartwright, P., Muller, H., Wagener, C., Holm, K., and Helin, K. (1998). E2F-6, a novel member of the E2F family is an inhibitor of E2F-dependent transcription. Oncogene 17, 611-623. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

