Nuclear translocation of integrin cytoplasmic domain-associated protein 1 stimulates cellular proliferation
- PMID: 15703214
- PMCID: PMC1073667
- DOI: 10.1091/mbc.e04-08-0744
Nuclear translocation of integrin cytoplasmic domain-associated protein 1 stimulates cellular proliferation
Abstract
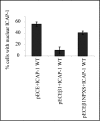
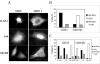
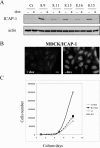
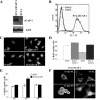
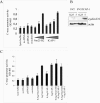
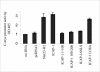
Integrin cytoplasmic domain-associated protein 1 (ICAP-1) has been shown to interact specifically with the beta1 integrin cytoplasmic domain and to control cell spreading on fibronectin. Interestingly, ICAP-1 also is observed in the nucleus, by immunocytochemical staining, and after biochemical cell fractionation, suggesting that it has additional roles that have yet to be determined. We show that the nucleocytoplasmic shuttling capability of ICAP-1 is dependent on a functional nuclear localization signal. In addition, overexpression of beta1 integrin strongly reduced this nuclear localization, suggesting that integrin activity could modulate ICAP-1 shuttling by sequestering it in the cytoplasm. Indeed, the nuclear localization of ICAP-1 is dependent on the stage of cell spreading on fibronectin, and we also show that ICAP-1 expression stimulates cellular proliferation in a fibronectin-dependent manner. This function is dependent on its nuclear localization. Moreover, ICAP-1 is able to activate the c-myc promoter in vitro. Together, these results demonstrate that ICAP-1 shuttles between the nucleus and cytoplasm in a beta1 integrin-dependent manner. It could act as a messenger that relays information from sites of integrin-dependent cell adhesion to the nucleus for controlling gene expression and cell proliferation.
Figures



Similar articles
-
Calcium and calmodulin-dependent serine/threonine protein kinase type II (CaMKII)-mediated intramolecular opening of integrin cytoplasmic domain-associated protein-1 (ICAP-1α) negatively regulates β1 integrins.J Biol Chem. 2013 Jul 12;288(28):20248-60. doi: 10.1074/jbc.M113.455956. Epub 2013 May 28. J Biol Chem. 2013. PMID: 23720740 Free PMC article.
-
Nuclear Localization of Integrin Cytoplasmic Domain-associated Protein-1 (ICAP1) Influences β1 Integrin Activation and Recruits Krev/Interaction Trapped-1 (KRIT1) to the Nucleus.J Biol Chem. 2017 Feb 3;292(5):1884-1898. doi: 10.1074/jbc.M116.762393. Epub 2016 Dec 21. J Biol Chem. 2017. PMID: 28003363 Free PMC article.
-
Interaction of the integrin beta1 cytoplasmic domain with ICAP-1 protein.J Biol Chem. 1999 Jan 1;274(1):11-9. doi: 10.1074/jbc.274.1.11. J Biol Chem. 1999. PMID: 9867804
-
Concepts and hypothesis: integrin cytoplasmic domain-associated protein-1 (ICAP-1) as a potential player in cerebral cavernous malformation.J Neurol. 2013 Jan;260(1):10-9. doi: 10.1007/s00415-012-6567-6. Epub 2012 Jun 19. J Neurol. 2013. PMID: 22711159 Review.
-
Unraveling ICAP-1 function: toward a new direction?Eur J Cell Biol. 2006 Apr;85(3-4):275-82. doi: 10.1016/j.ejcb.2005.10.005. Epub 2005 Nov 16. Eur J Cell Biol. 2006. PMID: 16546571 Review.
Cited by
-
Cerebral cavernous malformation: new molecular and clinical insights.J Med Genet. 2006 Sep;43(9):716-21. doi: 10.1136/jmg.2006.041079. Epub 2006 Mar 29. J Med Genet. 2006. PMID: 16571644 Free PMC article. Review.
-
Recent insights into cerebral cavernous malformations: a complex jigsaw puzzle under construction.FEBS J. 2010 Mar;277(5):1084-96. doi: 10.1111/j.1742-4658.2009.07537.x. Epub 2010 Jan 22. FEBS J. 2010. PMID: 20096036 Free PMC article. Review.
-
ARL4D recruits cytohesin-2/ARNO to modulate actin remodeling.Mol Biol Cell. 2007 Nov;18(11):4420-37. doi: 10.1091/mbc.e07-02-0149. Epub 2007 Sep 5. Mol Biol Cell. 2007. PMID: 17804820 Free PMC article.
-
Molecular features contributing to virus-independent intracellular localization and dynamic behavior of the herpesvirus transport protein US9.PLoS One. 2014 Aug 18;9(8):e104634. doi: 10.1371/journal.pone.0104634. eCollection 2014. PLoS One. 2014. PMID: 25133647 Free PMC article.
-
ITGB1BP1, a Novel Transcriptional Target of CD44-Downstream Signaling Promoting Cancer Cell Invasion.Breast Cancer (Dove Med Press). 2023 May 24;15:373-380. doi: 10.2147/BCTT.S404565. eCollection 2023. Breast Cancer (Dove Med Press). 2023. PMID: 37252376 Free PMC article. Review.
References
-
- Barberis, L., Wary, K. K., Fiucci, G., Liu, F., Hirsch, E., Brancaccio, M., Altruda, F., Tarone, G., and Giancotti, F. G. (2000). Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J. Biol. Chem. 275, 36532-36540. - PubMed
-
- Barone, M. V., and Courtneidge, S. A. (1995). Myc but not Fos rescue of platelet-derived growth factor signalling block caused by kinase-inactive Src. Nature 378, 509-512. - PubMed
-
- Benaud, C. M., and Dickson, R. B. (2001). Regulation of the expression of c-Myc by β1 integrins in epithelial cells. Oncogene 20, 759-768. - PubMed
-
- Ben-Ze'ev, A., Shtutman, M., and Zhurinsky, J. (2000). The integration of cell adhesion with gene expression: the role of β-catenin. Exp. Cell Res. 261, 75-82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

