The microtubule-associated protein tumor overexpressed gene binds to the RNA trafficking protein heterogeneous nuclear ribonucleoprotein A2
- PMID: 15703215
- PMCID: PMC1073673
- DOI: 10.1091/mbc.e04-08-0709
The microtubule-associated protein tumor overexpressed gene binds to the RNA trafficking protein heterogeneous nuclear ribonucleoprotein A2
Abstract
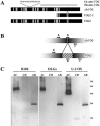
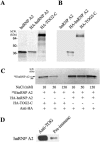
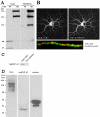
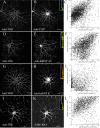
In neural cells, such as oligodendrocytes and neurons, transport of certain RNAs along microtubules is mediated by the cis-acting heterogeneous nuclear ribonucleoprotein A2 response element (A2RE) trafficking element and the cognate trans-acting heterogeneous nuclear ribonucleoprotein (hnRNP) A2 trafficking factor. Using a yeast two-hybrid screen, we have identified a microtubule-associated protein, tumor overexpressed gene (TOG)2, as an hnRNP A2 binding partner. The C-terminal third of TOG2 is sufficient for hnRNP A2 binding. TOG2, the large protein isoform of TOG, is the only isoform detected in oligodendrocytes in culture. TOG coimmunoprecipitates with hnRNP A2 present in the cytoskeleton (CSK) fraction of neural cells, and both coprecipitate with microtubule stabilized pellets. Staining with anti-TOG reveals puncta that are localized in proximity to microtubules, often at the plus ends. TOG is colocalized with hnRNP A2 and A2RE-mRNA in trafficking granules that remain associated with CSK-insoluble tissue. These data suggest that TOG mediates the association of hnRNP A2-positive granules with microtubules during transport and/or localization.
Figures
Similar articles
-
Heterogeneous nuclear ribonucleoprotein (hnRNP) E1 binds to hnRNP A2 and inhibits translation of A2 response element mRNAs.Mol Biol Cell. 2006 Aug;17(8):3521-33. doi: 10.1091/mbc.e05-10-0946. Epub 2006 Jun 14. Mol Biol Cell. 2006. PMID: 16775011 Free PMC article.
-
The microtubule-associated protein tumor overexpressed gene/cytoskeleton-associated protein 5 is necessary for myelin basic protein expression in oligodendrocytes.J Neurosci. 2007 Jul 18;27(29):7654-62. doi: 10.1523/JNEUROSCI.0203-07.2007. J Neurosci. 2007. PMID: 17634360 Free PMC article.
-
Rules of engagement promote polarity in RNA trafficking.BMC Neurosci. 2006 Oct 30;7 Suppl 1(Suppl 1):S3. doi: 10.1186/1471-2202-7-S1-S3. BMC Neurosci. 2006. PMID: 17118157 Free PMC article. Review.
-
RNA trafficking signals in human immunodeficiency virus type 1.Mol Cell Biol. 2001 Mar;21(6):2133-43. doi: 10.1128/MCB.21.6.2133-2143.2001. Mol Cell Biol. 2001. PMID: 11238947 Free PMC article.
-
Systems analysis of RNA trafficking in neural cells.Biol Cell. 2005 Jan;97(1):51-62. doi: 10.1042/BC20040083. Biol Cell. 2005. PMID: 15601257 Review.
Cited by
-
Making myelin basic protein -from mRNA transport to localized translation.Front Cell Neurosci. 2013 Sep 27;7:169. doi: 10.3389/fncel.2013.00169. Front Cell Neurosci. 2013. PMID: 24098271 Free PMC article. Review.
-
Conditional knockout of TOG results in CNS hypomyelination.Glia. 2017 Mar;65(3):489-501. doi: 10.1002/glia.23106. Epub 2017 Jan 7. Glia. 2017. PMID: 28063167 Free PMC article.
-
Role of calcineurin, hnRNPA2 and Akt in mitochondrial respiratory stress-mediated transcription activation of nuclear gene targets.Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1055-65. doi: 10.1016/j.bbabio.2010.02.008. Epub 2010 Feb 11. Biochim Biophys Acta. 2010. PMID: 20153290 Free PMC article.
-
Tyrosine phosphorylation regulates hnRNPA2 granule protein partitioning and reduces neurodegeneration.EMBO J. 2021 Feb 1;40(3):e105001. doi: 10.15252/embj.2020105001. Epub 2020 Dec 22. EMBO J. 2021. PMID: 33349959 Free PMC article.
-
Multiplexed dendritic targeting of alpha calcium calmodulin-dependent protein kinase II, neurogranin, and activity-regulated cytoskeleton-associated protein RNAs by the A2 pathway.Mol Biol Cell. 2008 May;19(5):2311-27. doi: 10.1091/mbc.e07-09-0914. Epub 2008 Feb 27. Mol Biol Cell. 2008. PMID: 18305102 Free PMC article.
References
-
- Andrade, M. A., Petosa, C., O'Donoghue, S. I., Muller, C. W., and Bork, P. (2001). Comparison of ARM and HEAT protein repeats. J. Mol. Biol. 309, 1-18. - PubMed
-
- Antic, D., and Keene, J. D. (1998). Messenger ribonucleoprotein complexes containing human ELAV proteins: interactions with cytoskeleton and translational apparatus. J. Cell Sci. 111, 183-197. - PubMed
-
- Barbarese, E., Brumwell, C., Kwon, S., Cui, H., and Carson, J. H. (1999). RNA on the road to myelin. J. Neurocytol. 4–5, 263-270. - PubMed
-
- Becker, B. E., and Gard, D. L. (2000). Multiple isoforms of the high molecular weight microtubule associated protein XMAP215 are expressed during development in Xenopus. Cell Motil. Cytoskeleton 47, 282-295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

