Sequestration of pRb by cyclin D3 causes intranuclear reorganization of lamin A/C during muscle cell differentiation
- PMID: 15703219
- PMCID: PMC1073674
- DOI: 10.1091/mbc.e04-02-0154
Sequestration of pRb by cyclin D3 causes intranuclear reorganization of lamin A/C during muscle cell differentiation
Abstract
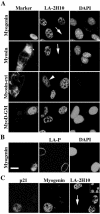
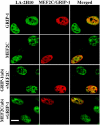
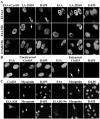
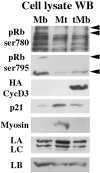
The A-type lamins that localize in nuclear domains termed lamin speckles are reorganized and antigenically masked specifically during myoblast differentiation. This rearrangement was observed to be linked to the myogenic program as lamin speckles, stained with monoclonal antibody (mAb) LA-2H10, were reorganized in MyoD-transfected fibroblasts induced to transdifferentiate to muscle cells. In C2C12 myoblasts, speckles were reorganized early during differentiation in cyclin D3-expressing cells. Ectopic cyclin D3 induced lamin reorganization in C2C12 myoblasts but not in other cell types. Experiments with adenovirus E1A protein that can bind to and segregate the retinoblastoma protein (pRb) indicated that pRb was essential for the cyclin D3-mediated reorganization of lamin speckles. Cyclin D3-expressing myoblasts displayed site-specific reduction of pRb phosphorylation. Furthermore, disruption of lamin structures by overexpression of lamins inhibited expression of the muscle regulatory factor myogenin. Our results suggest that the reorganization of internal lamins in muscle cells is mediated by key regulators of the muscle differentiation program.
Figures




Similar articles
-
pRb-dependent cyclin D3 protein stabilization is required for myogenic differentiation.Mol Cell Biol. 2007 Oct;27(20):7248-65. doi: 10.1128/MCB.02199-06. Epub 2007 Aug 20. Mol Cell Biol. 2007. PMID: 17709384 Free PMC article.
-
Differentiation of C2C12 myoblasts expressing lamin A mutated at a site responsible for Emery-Dreifuss muscular dystrophy is improved by inhibition of the MEK-ERK pathway and stimulation of the PI3-kinase pathway.Exp Cell Res. 2008 Apr 1;314(6):1392-405. doi: 10.1016/j.yexcr.2008.01.018. Epub 2008 Feb 6. Exp Cell Res. 2008. PMID: 18294630
-
Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblast differentiation.Mol Cell Biol. 1999 Jul;19(7):5203-17. doi: 10.1128/MCB.19.7.5203. Mol Cell Biol. 1999. PMID: 10373569 Free PMC article.
-
Distinct changes in intranuclear lamin A/C organization during myoblast differentiation.J Cell Sci. 2001 Nov;114(Pt 22):4001-11. doi: 10.1242/jcs.114.22.4001. J Cell Sci. 2001. PMID: 11739632
-
Synergistic up-regulation of muscle LIM protein expression in C2C12 and NIH3T3 cells by myogenin and MEF2C.Mol Genet Genomics. 2009 Jan;281(1):1-10. doi: 10.1007/s00438-008-0393-7. Epub 2008 Nov 6. Mol Genet Genomics. 2009. PMID: 18987887 Review.
Cited by
-
A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C.Genes Dev. 2006 Feb 1;20(3):307-20. doi: 10.1101/gad.349506. Genes Dev. 2006. PMID: 16452503 Free PMC article.
-
Regulation of lamin properties and functions: does phosphorylation do it all?Open Biol. 2015 Nov;5(11):150094. doi: 10.1098/rsob.150094. Open Biol. 2015. PMID: 26581574 Free PMC article. Review.
-
Cyclin D3 Colocalizes with Myogenin and p21 in Skeletal Muscle Satellite Cells during Early-Stage Functional Overload.Acta Histochem Cytochem. 2023 Dec 28;56(6):111-119. doi: 10.1267/ahc.23-00041. Epub 2023 Dec 20. Acta Histochem Cytochem. 2023. PMID: 38318102 Free PMC article.
-
Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin.Genes Dev. 2008 Apr 1;22(7):832-53. doi: 10.1101/gad.1652708. Genes Dev. 2008. PMID: 18381888 Free PMC article. Review.
-
Laminopathies: multiple disorders arising from defects in nuclear architecture.J Biosci. 2006 Sep;31(3):405-21. doi: 10.1007/BF02704113. J Biosci. 2006. PMID: 17006023 Review.
References
-
- Bartkova, J., Lukas, J., Strauss, M., and Bartek, J. (1998). Cyclin D3, requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 17, 1027-1037. - PubMed
-
- Black, B. L., and Olson, E. N. (1998). Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 14, 167-196. - PubMed
-
- Blau, H. M., Chiu, C. P., and Webster, C. (1983). Cytoplasmic activation of human nuclear genes in stable heterokaryons. Cell 32, 1171-1180. - PubMed
-
- Bonne, G. et al. (1999). Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 21, 285-288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

