Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells
- PMID: 15703291
- PMCID: PMC549508
- DOI: 10.1073/pnas.0500014102
Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells
Abstract
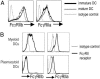
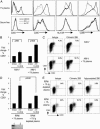
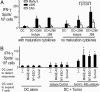
The final differentiation or maturation of dendritic cells (DCs) in response to environmental stimuli influences their ability to both initiate immunity and determine the quality of the response to antigens. Circulating immune complexes and cell-bound immunoglobulins present in normal human sera represent a potential stimulus for inadvertent DC activation in the steady state and during autoimmunity. Here, we show that selective blockade of the inhibitory Fcgamma receptor (FcgammaR) FcgammaRIIb with recently developed monoclonal antibodies leads to maturation of human monocyte-derived DCs, which depends on the presence of IgG in normal human plasma. Plasma, in the presence of an FcgammaRIIb blockade, caused the DCs to up-regulate the expression of costimulatory molecules and to produce the inflammatory mediator IL-12p70. FcgammaRIIb blockade of DCs loaded with tumor cells led to increased tumor-specific T cell immunity without the need for exogenous stimuli other than human plasma. Therefore, the activation status of DCs in the presence of normal human serum depends on the balance between activating and inhibitory FcgammaRs and can be enhanced by new antibodies that react selectively with FcgammaRIIb. These data suggest an approach for modifying this balance to enhance immunity to immune complexes and antibody-coated tumor cells and to silence DC activation by immune complexes in autoimmune states.
Figures
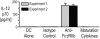

References
-
- Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. (2003) Annu. Rev. Immunol. 21, 685–711. - PubMed
-
- Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y. J., Pulendran, B. & Palucka, K. (2000) Annu. Rev. Immunol. 18, 767–811. - PubMed
-
- Mellman, I. & Steinman, R. M. (2001) Cell 106, 255–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

