Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain
- PMID: 15703292
- PMCID: PMC549507
- DOI: 10.1073/pnas.0500012102
Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain
Abstract
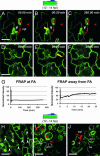
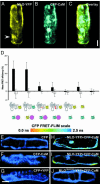
Many fungal pathogens must enter plant cells for successful colonization. Barley mildew resistance locus o (Mlo) is required for host cell invasion upon attack by the ascomycete powdery mildew fungus, Blumeria graminis f.sp. hordei, and encodes the founder of a family of heptahelical integral membrane proteins unique to plants. Recessively inherited loss-of-function mutant alleles (mlo) result in effective penetration resistance to all isolates of the biotrophic parasite. We used noninvasive fluorescence-based imaging to show that fluorescently tagged MLO protein becomes redistributed in the plasma membrane (PM) and accumulates beneath fungal appressoria coincident with the initiation of pathogen entry into host cells. Polarized MLO accumulation occurs once upon attack and appears to be independent of actin cytoskeleton function. Likewise, barley ROR2 syntaxin, a genetically defined component of penetration resistance to B. graminis f.sp. hordei, and a subset of predicted PM-resident proteins become redistributed to fungal entry sites. We previously identified calmodulin, a cytoplasmic calcium sensor, as an interactor and positive regulator of MLO activity and demonstrate here by FRET microscopy an increase in MLO/calmodulin FRET around penetration sites coincident with successful host cell entry. Our data provide evidence for the formation of a pathogen-triggered PM microdomain that is reminiscent of membrane microdomains (lipid rafts) induced upon attempted entry of pathogenic bacteria in animal cells.
Figures


References
-
- Mengaud, J., Ohayon, H., Gounon, P., Mege, R. M. & Cossart, P. (1996) Cell 84, 923-932. - PubMed
-
- Panstruga, R. & Schulze-Lefert, P. (2003) Microbes Infect. 5, 429-437. - PubMed
-
- Büschges, R., Hollricher, K., Panstruga, R., Simons, G., Wolter, M., Frijters, A., van Daelen, R., van der Lee, T., Diergaarde, P., Groenendijk, J., et al. (1997) Cell 88, 695-705. - PubMed
-
- Devoto, A., Piffanelli, P., Nilsson, I., Wallin, E., Panstruga, R., von Heijne, G. & Schulze-Lefert, P. (1999) J. Biol. Chem. 274, 34993-35004. - PubMed
-
- Collins, N. C., Thordal-Christensen, H., Lipka, V., Bau, S., Kombrink, E., Qiu, J. L., Hückelhoven, R., Stein, M., Freialdenhoven, A., Somerville, S. C. & Schulze-Lefert, P. (2003) Nature 425, 973-977. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

