Transcellular induction of neuropeptide Y expression by NT4 and BDNF
- PMID: 15703301
- PMCID: PMC549439
- DOI: 10.1073/pnas.0404712102
Transcellular induction of neuropeptide Y expression by NT4 and BDNF
Abstract
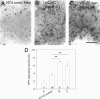
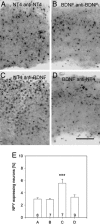
The transcellular signaling of neurotrophins is postulated, but evidence is scarce. We now show that a small number of NT4- and BDNF-overexpressing neurons in the cortical explant of thalamocortical cocultures rapidly evoked a Trk receptor-dependent upregulation of neuropeptide Y (NPY) mRNA in interneurons. In contrast to BDNF, the action of NT4 was independent of calcium influx through NMDA receptors and L-type calcium channels. NPY neurons vastly outnumbered the neurotrophin-overexpressing neurons (mostly pyramidal cells), arguing for a spread of the neurotrophin signal via axonally connected neuronal populations. Furthermore, NT4 transfection of one explant of axonally connected corticocortical cocultures evoked significantly larger numbers of NPY neurons in both explants. Delivery of the signal was not by diffusion of neurotrophins via the medium. Moreover, cortical NPY neuron numbers increased after NT4 and BDNF transfection of a cocultured tectal explant innervated selectively by cortical layer V pyramidal neurons. The transcellular induction of NPY suggests a source-to-sink model for axonal transport and a local cortical redistribution of TrkB ligands to interneurons competent for NPY expression.
Figures
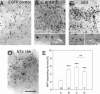
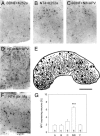

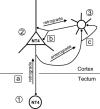
 ) become a source of NT4. Nonoverexpressing pyramidal neurons in layer V of the cortical explant (
) become a source of NT4. Nonoverexpressing pyramidal neurons in layer V of the cortical explant ( ) represent a first sink of NT4 and retrogradely import the factor (a). Now, layer V pyramidal neurons (
) represent a first sink of NT4 and retrogradely import the factor (a). Now, layer V pyramidal neurons ( ) accumulate NT4 and represent a source of NT4 for cortical interneurons (
) accumulate NT4 and represent a source of NT4 for cortical interneurons ( ). They, in turn, represent the second sink of NT4 and either retrieve somatic or dendritic released imported NT4 (b) or receive imported NT4 by anterograde delivery via pyramidal cell axon collaterals (c). The model also explains the results obtained in corticocortical cocultures; in addition, transfected projection neurons may directly deliver NT4 anterogradely to neurons in nontransfected explants.
). They, in turn, represent the second sink of NT4 and either retrieve somatic or dendritic released imported NT4 (b) or receive imported NT4 by anterograde delivery via pyramidal cell axon collaterals (c). The model also explains the results obtained in corticocortical cocultures; in addition, transfected projection neurons may directly deliver NT4 anterogradely to neurons in nontransfected explants.Similar articles
-
Accelerated dendritic development of rat cortical pyramidal cells and interneurons after biolistic transfection with BDNF and NT4/5.Development. 2003 Dec;130(23):5827-38. doi: 10.1242/dev.00826. Development. 2003. PMID: 14573511
-
Comparison of neurotrophin regulation of human and rat neuropeptide Y (NPY) neurons: induction of NPY production in aggregate cultures derived from rat but not from human fetal brains.Brain Res. 1996 Sep 2;732(1-2):52-60. doi: 10.1016/0006-8993(96)00486-6. Brain Res. 1996. PMID: 8891268
-
Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures.Eur J Neurosci. 1999 Apr;11(4):1179-90. doi: 10.1046/j.1460-9568.1999.00551.x. Eur J Neurosci. 1999. PMID: 10103114
-
Neurotrophin regulation of the developing nervous system: analyses of knockout mice.Rev Neurosci. 1997 Jan-Mar;8(1):13-27. doi: 10.1515/revneuro.1997.8.1.13. Rev Neurosci. 1997. PMID: 9402642 Review.
-
Neurotrophins and activity-dependent plasticity of cortical interneurons.Trends Neurosci. 1997 May;20(5):198-202. doi: 10.1016/s0166-2236(96)01026-0. Trends Neurosci. 1997. PMID: 9141194 Review.
Cited by
-
Estrogen and Serotonin: Complexity of Interactions and Implications for Epileptic Seizures and Epileptogenesis.Curr Neuropharmacol. 2019;17(3):214-231. doi: 10.2174/1570159X16666180628164432. Curr Neuropharmacol. 2019. PMID: 29956631 Free PMC article. Review.
-
G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function.J Physiol. 2012 Mar 15;590(6):1313-30. doi: 10.1113/jphysiol.2011.222794. Epub 2011 Dec 19. J Physiol. 2012. PMID: 22183719 Free PMC article. Review.
-
The physiology of regulated BDNF release.Cell Tissue Res. 2020 Oct;382(1):15-45. doi: 10.1007/s00441-020-03253-2. Epub 2020 Sep 18. Cell Tissue Res. 2020. PMID: 32944867 Free PMC article. Review.
-
ABCA7-dependent induction of neuropeptide Y is required for synaptic resilience in Alzheimer's disease through BDNF/NGFR signaling.Cell Genom. 2024 Sep 11;4(9):100642. doi: 10.1016/j.xgen.2024.100642. Epub 2024 Aug 30. Cell Genom. 2024. PMID: 39216475 Free PMC article.
-
Sex differences in the neurobiology of epilepsy: a preclinical perspective.Neurobiol Dis. 2014 Dec;72 Pt B:180-92. doi: 10.1016/j.nbd.2014.07.004. Epub 2014 Jul 21. Neurobiol Dis. 2014. PMID: 25058745 Free PMC article. Review.
References
-
- Lessmann, V., Gottmann, K. & Malcangio, M. (2003) Prog. Neurobiol. 69, 341-374. - PubMed
-
- Altar, C. A. & DiStefano, P. S. (1998) Trends Neurosci. 21, 433-437. - PubMed
-
- Riddle, D. R., Lo, D. C. & Katz, L. C. (1995) Nature 378, 189-191. - PubMed
-
- Spalding, K. L., Tan, M. M., Hendry, I. A. & Harvey, A. R. (2002) Mol. Cell. Neurosci. 19, 485-500. - PubMed
-
- Wahle, P., Di Cristo, G., Schwerdtfeger, G., Engelhardt, M., Berardi, N. & Maffei, L. (2003) Development (Cambridge, U.K.) 130, 611-622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

