Sequential-context-dependent hippocampal activity is not necessary to learn sequences with repeated elements
- PMID: 15703385
- PMCID: PMC6725980
- DOI: 10.1523/JNEUROSCI.2901-04.2005
Sequential-context-dependent hippocampal activity is not necessary to learn sequences with repeated elements
Abstract
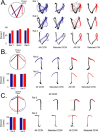
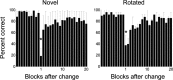
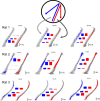
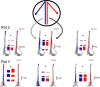
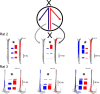
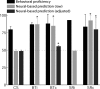
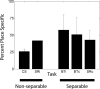
Learning sequences of events (e.g., a-b-c) is conceptually a simple problem that can be solved using asymmetrically linked cell assemblies [e.g., "phase sequences" (Hebb, 1949)], provided that the elements of the sequence are unique. When elements repeat within the sequence, however (e.g., a-b-c-d-b-e), the same element belongs to two separate "contexts," and a more complex sequence encoding mechanism is required to differentiate between the two contexts. Some neural structure must form sequential-context-dependent, or "differential," representations of the two contexts (i.e., b as an element of "a-b-c" as opposed to "d-b-e") to allow the correct choice to be made after the repeated element. To investigate the possible role of hippocampus in complex sequence encoding, rats were trained to remember repeated-location sequences under three conditions: (1) reward was given at each location; (2) during training, moveable barriers were placed at the entry and exit of the repeated segment to direct the rat and were removed once the sequence was learned; and (3) reward was withheld at the entry and exit of the repeated segment. In the first condition, hippocampal ensemble activity did not differentiate the sequential context of the repeated segment, indicating that complex sequences with repeated segments can be learned without differential encoding within the hippocampus. Differential hippocampal encoding was observed, however, under the latter two conditions, suggesting that long-term memory for discriminative cues present only during training, working memory of the most recently visited reinforcement sites, or anticipation of the subsequent reinforcement site can separate hippocampal activity patterns at the same location.
Figures


Similar articles
-
Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability.J Neurosci. 2006 Dec 20;26(51):13143-55. doi: 10.1523/JNEUROSCI.3803-06.2006. J Neurosci. 2006. PMID: 17182765 Free PMC article.
-
The effects of AMPA-induced lesions of the septo-hippocampal cholinergic projection on aversive conditioning to explicit and contextual cues and spatial learning in the water maze.Eur J Neurosci. 1995 Feb 1;7(2):281-92. doi: 10.1111/j.1460-9568.1995.tb01064.x. Eur J Neurosci. 1995. PMID: 7538856
-
Parallel processing of information about location in the amygdala, entorhinal cortex and hippocampus.Hippocampus. 2013 Nov;23(11):1075-83. doi: 10.1002/hipo.22179. Hippocampus. 2013. PMID: 23929819 Review.
-
Disruption of delayed memory for a sequence of spatial locations following CA1- or CA3-lesions of the dorsal hippocampus.Neurobiol Learn Mem. 2005 Sep;84(2):138-47. doi: 10.1016/j.nlm.2005.06.002. Neurobiol Learn Mem. 2005. PMID: 16054848
-
Parallel processing across neural systems: implications for a multiple memory system hypothesis.Neurobiol Learn Mem. 2004 Nov;82(3):278-98. doi: 10.1016/j.nlm.2004.07.007. Neurobiol Learn Mem. 2004. PMID: 15464410 Review.
Cited by
-
Spatial representation in the hippocampal formation: a history.Nat Neurosci. 2017 Oct 26;20(11):1448-1464. doi: 10.1038/nn.4653. Nat Neurosci. 2017. PMID: 29073644 Review.
-
Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination.Hippocampus. 2012 May;22(5):1121-33. doi: 10.1002/hipo.20958. Epub 2011 May 31. Hippocampus. 2012. PMID: 21630374 Free PMC article.
-
Remapping and realignment in the human hippocampal formation predict context-dependent spatial behavior.Nat Neurosci. 2021 Jun;24(6):863-872. doi: 10.1038/s41593-021-00835-3. Epub 2021 Apr 15. Nat Neurosci. 2021. PMID: 33859438
-
Reactivation of Rate Remapping in CA3.J Neurosci. 2016 Sep 7;36(36):9342-50. doi: 10.1523/JNEUROSCI.1678-15.2016. J Neurosci. 2016. PMID: 27605610 Free PMC article.
-
An Integrated Platform for In Vivo Electrophysiology in Spatial Cognition Experiments.eNeuro. 2023 Nov 21;10(11):ENEURO.0274-23.2023. doi: 10.1523/ENEURO.0274-23.2023. Print 2023 Nov. eNeuro. 2023. PMID: 37989581 Free PMC article.
References
-
- Abbott LF, Blum KI (1996) Functional significance of long-term potentiation for sequence learning and prediction. Cereb Cortex 6: 406-416. - PubMed
-
- Ainge JA, Wood E (2003) Excitotoxic lesions of the hippocampus impair performance on a continuous alternation T-maze task with short delays but not with no delay. Soc Neurosci Abstr 29: 91.1.
-
- Bostock E, Muller RU, Kubie JL (1991) Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1: 193-205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
