Altered ion channels in an animal model of Charcot-Marie-Tooth disease type IA
- PMID: 15703401
- PMCID: PMC6726003
- DOI: 10.1523/JNEUROSCI.3328-04.2005
Altered ion channels in an animal model of Charcot-Marie-Tooth disease type IA
Abstract
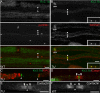
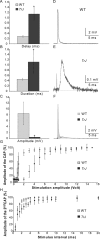
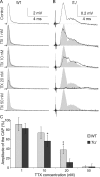
How demyelination and remyelination affect the function of myelinated axons is a fundamental aspect of demyelinating diseases. We examined this issue in Trembler-J mice, a genetically authentic model of a dominantly inherited demyelinating neuropathy of humans. The K+ channels Kv1.1 and Kv1.2 channels were often improperly located in the paranodal axon membrane, typically associated with improperly formed paranodes, and in unmyelinated segments between internodes. As in wild-type nerves, Trembler-J nodes contained Nav1.6, ankyrin-G, betaIV-spectrin, and KCNQ2, but, unlike wild-type nerves, they also contained Kv3.1b and Nav1.8. In unmyelinated segments bordered by myelin sheaths, these proteins were clustered in heminodes and did not appear to be diffusely localized in the unmyelinated segments themselves. Nodes and heminodes were contacted by Schwann cells processes that did not have the ultrastructural or molecular characteristics of mature microvilli. Despite the presence of Nav1.8, a tetrodotoxin-resistant sodium channel, sciatic nerve conduction was at least as sensitive to tetrodotoxin in Trembler-J nerves as in wild-type nerves. Thus, the profound reorganization of axonal ion channels and the aberrant expression of novel ion channels likely contribute to the altered conduction in Trembler-J nerves.
Figures
Similar articles
-
Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier.J Neurosci. 2003 Aug 6;23(18):7001-11. doi: 10.1523/JNEUROSCI.23-18-07001.2003. J Neurosci. 2003. PMID: 12904461 Free PMC article.
-
Altered expression of ion channel isoforms at the node of Ranvier in P0-deficient myelin mutants.Mol Cell Neurosci. 2004 Jan;25(1):83-94. doi: 10.1016/j.mcn.2003.09.015. Mol Cell Neurosci. 2004. PMID: 14962742
-
Normal nerve striations are altered in the trembler-J mouse, a model of Charcot-Marie-Tooth disease.Muscle Nerve. 2015 Feb;51(2):246-52. doi: 10.1002/mus.24303. Muscle Nerve. 2015. PMID: 24890015
-
Molecular dissection of the myelinated axon.Ann Neurol. 1993 Feb;33(2):121-36. doi: 10.1002/ana.410330202. Ann Neurol. 1993. PMID: 7679565 Review.
-
Molecular specializations at nodes and paranodes in peripheral nerve.Microsc Res Tech. 1996 Aug 1;34(5):452-61. doi: 10.1002/(SICI)1097-0029(19960801)34:5<452::AID-JEMT5>3.0.CO;2-O. Microsc Res Tech. 1996. PMID: 8837021 Review.
Cited by
-
Axonal Spectrins: Nanoscale Organization, Functional Domains and Spectrinopathies.Front Cell Neurosci. 2019 May 28;13:234. doi: 10.3389/fncel.2019.00234. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31191255 Free PMC article. Review.
-
Mechanisms of node of Ranvier assembly.Nat Rev Neurosci. 2021 Jan;22(1):7-20. doi: 10.1038/s41583-020-00406-8. Epub 2020 Nov 25. Nat Rev Neurosci. 2021. PMID: 33239761 Review.
-
Intermediate forms of Charcot-Marie-Tooth neuropathy: a review.Neuromolecular Med. 2006;8(1-2):123-30. doi: 10.1385/nmm:8:1-2:123. Neuromolecular Med. 2006. PMID: 16775371 Review.
-
Familial trigeminal neuralgia - a systematic clinical study with a genomic screen of the neuronal electrogenisome.Cephalalgia. 2020 Jul;40(8):767-777. doi: 10.1177/0333102419897623. Epub 2020 Jan 13. Cephalalgia. 2020. PMID: 31928344 Free PMC article.
-
Neuro-glial interactions at the nodes of Ranvier: implication in health and diseases.Front Cell Neurosci. 2013 Oct 29;7:196. doi: 10.3389/fncel.2013.00196. Front Cell Neurosci. 2013. PMID: 24194699 Free PMC article. Review.
References
-
- Akopian AN, Sivilotti L, Wood JN (1996) A tetrodotoxin-resistant voltagegated sodium channel expressed by sensory neurons. Nature 379: 257-262. - PubMed
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN (1999) The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2: 541-548. - PubMed
-
- Arroyo EJ, Xu Y-T, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS (1999) Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvβ2, and Caspr. J Neurocytol 28: 333-347. - PubMed
-
- Bennett V, Lambert S, Davis JQ, Zhang X (1997) Molecular architecture of the specialized axonal membrane at the node of Ranvier. Soc Gen Physiol 52: 107-120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
