Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle
- PMID: 15703405
- PMCID: PMC6725981
- DOI: 10.1523/JNEUROSCI.4397-04.2005
Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle
Abstract
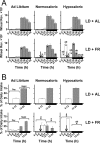
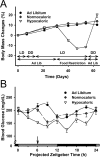
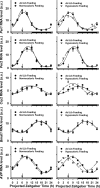
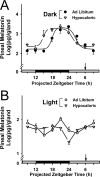
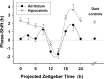
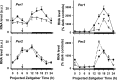
The suprachiasmatic nuclei (SCN) of the hypothalamus contain the master mammalian circadian clock, which is mainly reset by light. Temporal restricted feeding, a potent synchronizer of peripheral oscillators, has only weak influence on light-entrained rhythms via the SCN, unless restricted feeding is coupled with calorie restriction, thereby altering phase angle of photic synchronization. Effects of daytime restricted feeding were investigated on the mouse circadian system. Normocaloric feeding at midday led to a predominantly diurnal (60%) food intake and decreased blood glucose in the afternoon, but it did not affect the phase of locomotor activity rhythm or vasopressin expression in the SCN. In contrast, hypocaloric feeding at midday led to 2-4 h phase advances of three circadian outputs, locomotor activity rhythm, pineal melatonin, and vasopressin mRNA cycle in the SCN, and it decreased daily levels of blood glucose. Furthermore, Per1 and Cry2 oscillations in the SCN were phase advanced by 1 and 3 h, respectively, in hypocalorie- but not in normocalorie-fed mice. The phase of Per2 and Bmal1 expression remained unchanged regardless of feeding condition. Moreover, the shape of behavioral phase-response curve to light and light-induced expression of Per1 in the SCN were markedly modified in hypocalorie-fed mice compared with animals fed ad libitum. The present study shows that diurnal hypocaloric feeding affects not only the temporal organization of the SCN clockwork and circadian outputs in mice under light/dark cycle but also photic responses of the circadian system, thus indicating that energy metabolism modulates circadian rhythmicity and gating of photic inputs in mammals.
Figures




Similar articles
-
Synchronization of the molecular clockwork by light- and food-related cues in mammals.Biol Chem. 2003 May;384(5):711-9. doi: 10.1515/BC.2003.079. Biol Chem. 2003. PMID: 12817467 Review.
-
Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal Arvicanthis ansorgei.Neuroscience. 2003;116(2):583-91. doi: 10.1016/s0306-4522(02)00654-1. Neuroscience. 2003. PMID: 12559113
-
Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei.Neuroscience. 2003;120(3):745-55. doi: 10.1016/s0306-4522(03)00344-0. Neuroscience. 2003. PMID: 12895514
-
[Clock genes, circadian rhythms and food intake].Pathol Biol (Paris). 2007 Apr-May;55(3-4):176-7. doi: 10.1016/j.patbio.2006.12.005. Epub 2007 Apr 6. Pathol Biol (Paris). 2007. PMID: 17412526 French.
-
Molecular analysis of mammalian circadian rhythms.Annu Rev Physiol. 2001;63:647-76. doi: 10.1146/annurev.physiol.63.1.647. Annu Rev Physiol. 2001. PMID: 11181971 Review.
Cited by
-
Restricted Feeding Resets Endogenous Circadian Rhythm in Female Mice Under Constant Darkness.Neurosci Bull. 2021 Jul;37(7):1005-1009. doi: 10.1007/s12264-021-00669-w. Epub 2021 Mar 29. Neurosci Bull. 2021. PMID: 33779891 Free PMC article. No abstract available.
-
Circadian clocks for all meal-times: anticipation of 2 daily meals in rats.PLoS One. 2012;7(2):e31772. doi: 10.1371/journal.pone.0031772. Epub 2012 Feb 15. PLoS One. 2012. PMID: 22355393 Free PMC article.
-
Dim light at night disturbs the daily sleep-wake cycle in the rat.Sci Rep. 2016 Oct 20;6:35662. doi: 10.1038/srep35662. Sci Rep. 2016. PMID: 27762290 Free PMC article.
-
Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms.Sci Rep. 2016 May 12;6:25970. doi: 10.1038/srep25970. Sci Rep. 2016. PMID: 27170536 Free PMC article.
-
Brain response to calorie restriction.Cell Mol Life Sci. 2013 Sep;70(17):3157-70. doi: 10.1007/s00018-012-1223-y. Epub 2012 Dec 27. Cell Mol Life Sci. 2013. PMID: 23269433 Free PMC article. Review.
References
-
- Abe H, Honma S, Namihira M, Tanahashi Y, Ikeda M, Honma KI (1998) Circadian rhythm and light responsiveness of BMAL1 expression, a partner of mammalian clock gene Clock, in the suprachiasmatic nucleus of rats. Neurosci Lett 258: 93-96. - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC (1997) A differential response of two putative mammalian circadian regulators, mPer1 and mPer2, to light. Cell 91: 1055-1064. - PubMed
-
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC (2001) mPer1 and mPer2 are essential for normal resetting of the circadian clock. J Biol Rhythms 16: 100-104. - PubMed
-
- Challet E, Pevet P (2003) Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front Biosci 8: s246-s257. - PubMed
-
- Challet E, Pevet P, Vivien-Roels B, Malan A (1997) Phase-advanced daily rhythms of melatonin, body temperature, and locomotor activity in food-restricted rats fed during daytime. J Biol Rhythms 12: 65-79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
