Transgenic expression of human connexin32 in myelinating Schwann cells prevents demyelination in connexin32-null mice
- PMID: 15703409
- PMCID: PMC6725992
- DOI: 10.1523/JNEUROSCI.3082-04.2005
Transgenic expression of human connexin32 in myelinating Schwann cells prevents demyelination in connexin32-null mice
Abstract
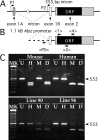
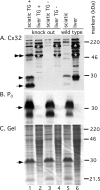
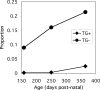
Mutations in Gap Junction beta1 (GJB1), the gene encoding the gap junction protein connexin32 (Cx32), cause the X-linked form of Charcot-Marie-Tooth disease (CMT1X), an inherited demyelinating neuropathy. We investigated the possibility that the expression of mutant Cx32 in other cells besides myelinating Schwann cells contributes to the development of demyelination. Human Cx32 was expressed in transgenic mice using a rat myelin protein zero (Mpz) promoter, which is exclusively expressed by myelinating Schwann cells. Male mice expressing the human transgene were crossed with female Gjb1/cx32-null mice; the resulting male offspring were all cx32-null (on the X chromosome), and one-half were transgene positive. In these transgenic mice, all of the Cx32 was derived from the expression of the transgene and was found in the sciatic nerve but not in the spinal cord or the liver. Furthermore, the Cx32 protein was properly localized (within incisures and paranodes) in myelinating Schwann cells. Finally, the expression of human Cx32 protein "rescued" the phenotype of cx32-null mice, because the transgenic mice have significantly fewer demyelinated or remyelinated axons than their nontransgenic littermates. These results indicate that the loss of Schwann-cell-autonomous expression of Cx32 is sufficient to account for demyelination in CMT1X.
Figures





Similar articles
-
Prenylation-defective human connexin32 mutants are normally localized and function equivalently to wild-type connexin32 in myelinating Schwann cells.J Neurosci. 2005 Aug 3;25(31):7111-20. doi: 10.1523/JNEUROSCI.1319-05.2005. J Neurosci. 2005. PMID: 16079393 Free PMC article.
-
The effects of a dominant connexin32 mutant in myelinating Schwann cells.Mol Cell Neurosci. 2006 Jul;32(3):283-98. doi: 10.1016/j.mcn.2006.05.001. Epub 2006 Jun 21. Mol Cell Neurosci. 2006. PMID: 16790356
-
Intraneural GJB1 gene delivery improves nerve pathology in a model of X-linked Charcot-Marie-Tooth disease.Ann Neurol. 2015 Aug;78(2):303-16. doi: 10.1002/ana.24441. Epub 2015 Jun 30. Ann Neurol. 2015. PMID: 26010264
-
How do mutations in GJB1 cause X-linked Charcot-Marie-Tooth disease?Brain Res. 2012 Dec 3;1487:198-205. doi: 10.1016/j.brainres.2012.03.068. Epub 2012 Jul 6. Brain Res. 2012. PMID: 22771394 Free PMC article. Review.
-
Molecular genetics of X-linked Charcot-Marie-Tooth disease.Neuromolecular Med. 2006;8(1-2):107-22. doi: 10.1385/nmm:8:1-2:107. Neuromolecular Med. 2006. PMID: 16775370 Review.
Cited by
-
Gap junction communication in myelinating glia.Biochim Biophys Acta. 2013 Jan;1828(1):69-78. doi: 10.1016/j.bbamem.2012.01.024. Epub 2012 Feb 3. Biochim Biophys Acta. 2013. PMID: 22326946 Free PMC article. Review.
-
Harmonized cross-species cell atlases of trigeminal and dorsal root ganglia.bioRxiv [Preprint]. 2023 Jul 5:2023.07.04.547740. doi: 10.1101/2023.07.04.547740. bioRxiv. 2023. Update in: Sci Adv. 2024 Jun 21;10(25):eadj9173. doi: 10.1126/sciadv.adj9173. PMID: 37461736 Free PMC article. Updated. Preprint.
-
AAV9-mediated Schwann cell-targeted gene therapy rescues a model of demyelinating neuropathy.Gene Ther. 2021 Nov;28(10-11):659-675. doi: 10.1038/s41434-021-00250-0. Epub 2021 Mar 10. Gene Ther. 2021. PMID: 33692503 Free PMC article.
-
Restoration of SMN in Schwann cells reverses myelination defects and improves neuromuscular function in spinal muscular atrophy.Hum Mol Genet. 2016 Jul 1;25(13):2853-2861. doi: 10.1093/hmg/ddw141. Epub 2016 May 11. Hum Mol Genet. 2016. PMID: 27170316 Free PMC article.
-
Pharmacologic Targeting of the C-Terminus of Heat Shock Protein 90 Improves Neuromuscular Function in Animal Models of Charcot Marie Tooth X1 Disease.ACS Pharmacol Transl Sci. 2023 Jan 20;6(2):306-319. doi: 10.1021/acsptsci.2c00223. eCollection 2023 Feb 10. ACS Pharmacol Transl Sci. 2023. PMID: 36798471 Free PMC article.
References
-
- Abel A, Bone LJ, Messing A, Scherer SS, Fischbeck KH (1999) Studies in transgenic mice indicate a loss of connexin32 function in X-linked Charcot-Marie-Tooth disease. J Neuropathol Exp Neurol 58: 702-710. - PubMed
-
- Bennett SAL, Arnold JM, Chen JH, Stenger J, Paul DL, Roberts DCS (1999) Long-term changes in connexin32 gap junction protein and mRNA expression following cocaine self-administration in rats. Eur J Neurosci 11: 3329-3338. - PubMed
-
- Bergoffen J, Scherer SS, Wang S, Oronzi-Scott M, Bone L, Paul DL, Chen K, Lensch MW, Chance P, Fischbeck K (1993) Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262: 2039-2042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
