Functions and mechanisms of receptor tyrosine kinase Torso signaling: lessons from Drosophila embryonic terminal development
- PMID: 15704136
- PMCID: PMC3092428
- DOI: 10.1002/dvdy.20295
Functions and mechanisms of receptor tyrosine kinase Torso signaling: lessons from Drosophila embryonic terminal development
Abstract
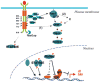
The Torso receptor tyrosine kinase (RTK) is required for cell fate specification in the terminal regions (head and tail) of the early Drosophila embryo. Torso contains a split tyrosine kinase domain and belongs to the type III subgroup of the RTK superfamily that also includes the platelet-derived growth factor receptors, stem cell or steel factor receptor c-Kit proto-oncoprotein, colony-stimulating factor-1 receptor, and vascular endothelial growth factor receptor. The Torso pathway has been a model system for studying RTK signal transduction. Genetic and biochemical studies of Torso signaling have provided valuable insights into the biological functions and mechanisms of RTK signaling during early Drosophila embryogenesis.
Copyright (c) 2005 Wiley-Liss, Inc.
Figures


References
-
- Agazie YM, Movilla N, Ischenko I, Hayman MJ. The phosphotyrosine phosphatase SHP2 is a critical mediator of transformation induced by the oncogenic fibroblast growth factor receptor 3. Oncogene. 2003;22:6909–6918. - PubMed
-
- Ambrosio L, Mahowald AP, Perrimon N. l(1)pole hole is required maternally for pattern formation in the terminal regions of the embryo. Development. 1989a;106:145–158. - PubMed
-
- Ambrosio L, Mahowald AP, Perrimon N. Requirement of the Drosophila raf homologue for torso function. Nature. 1989b;342:288–291. - PubMed
-
- Basler K, Hafen E. Receptor tyrosine kinases mediate cell–cell interactions during Drosophila development. Prog Growth Factor Res. 1990;2:15–27. - PubMed
-
- Bate M, Martinez Arias A. The development of Drosophila melanogaster. Vol. 2. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. p. 1558.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

