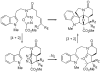Total synthesis of natural (+)- [corrected] and ent-(-)-4-desacetoxy-6,7-dihydrovindorosine [corrected] and natural and ent-minovine: oxadiazole tandem intramolecular Diels-Alder/1,3-dipolar cycloaddition reaction
- PMID: 15704939
- PMCID: PMC2587128
- DOI: 10.1021/ol050017s
Total synthesis of natural (+)- [corrected] and ent-(-)-4-desacetoxy-6,7-dihydrovindorosine [corrected] and natural and ent-minovine: oxadiazole tandem intramolecular Diels-Alder/1,3-dipolar cycloaddition reaction
Erratum in
- Org Lett. 2005 May 12;7(10):2079
Abstract
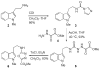
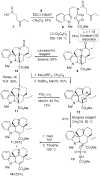
Efficient and unusually concise total syntheses of both enantiomers of the Aspidosperma alkaloids 4-desacetoxy-6,7-dihydrovindorosine (12) and minovine (1) are detailed. A tandem intramolecular Diels-Alder/1,3-dipolar cycloaddition reaction of the 1,3,4-oxadiazole 8, in which three new rings, four new C-C bonds, and five stereocenters are formed, is a key step in the sequence. The availability of optically active material permitted an assessment of the enantiomeric integrity of minovine and the source of its reported unusual optical rotation. [Reaction: see text]
Figures
Similar articles
-
Total synthesis of (-)- and ent-(+)-vindoline and related alkaloids.J Am Chem Soc. 2006 Aug 16;128(32):10596-612. doi: 10.1021/ja061256t. J Am Chem Soc. 2006. PMID: 16895428 Free PMC article.
-
Tandem Intramolecular Diels-Alder/1,3-Dipolar Cycloaddition Cascade of 1,3,4-Oxadiazoles: Initial Scope and Applications.Acc Chem Res. 2016 Feb 16;49(2):241-51. doi: 10.1021/acs.accounts.5b00510. Epub 2016 Jan 27. Acc Chem Res. 2016. PMID: 26813287 Free PMC article.
-
Total synthesis of anhydrolycorinone utilizing sequential intramolecular Diels-Alder reactions of a 1,3,4-oxadiazole.J Org Chem. 2002 Oct 18;67(21):7361-4. doi: 10.1021/jo020437k. J Org Chem. 2002. PMID: 12375965
-
Enabling syntheses of diterpenoid alkaloids and related diterpenes by an oxidative dearomatization/Diels-Alder cycloaddition strategy.Nat Prod Rep. 2017 Aug 30;34(9):1044-1050. doi: 10.1039/c7np00033b. Nat Prod Rep. 2017. PMID: 28799605 Review.
-
Total synthesis and biosynthesis of the paraherquamides: an intriguing story of the biological Diels-Alder construction.Chem Pharm Bull (Tokyo). 2002 Jun;50(6):711-40. doi: 10.1248/cpb.50.711. Chem Pharm Bull (Tokyo). 2002. PMID: 12045324 Review.
Cited by
-
Total synthesis and evaluation of vinblastine analogues containing systematic deep-seated modifications in the vindoline subunit ring system: core redesign.J Med Chem. 2013 Jan 24;56(2):483-95. doi: 10.1021/jm3014376. Epub 2013 Jan 4. J Med Chem. 2013. PMID: 23252481 Free PMC article.
-
Total synthesis of a key series of vinblastines modified at C4 that define the importance and surprising trends in activity.Chem Sci. 2017 Feb 1;8(2):1560-1569. doi: 10.1039/C6SC04146A. Epub 2016 Nov 3. Chem Sci. 2017. PMID: 28194270 Free PMC article.
-
Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues.J Am Chem Soc. 2009 Apr 8;131(13):4904-16. doi: 10.1021/ja809842b. J Am Chem Soc. 2009. PMID: 19292450 Free PMC article.
-
Asymmetric total synthesis of vindoline.J Am Chem Soc. 2010 Mar 24;132(11):3685-7. doi: 10.1021/ja910695e. J Am Chem Soc. 2010. PMID: 20187641 Free PMC article.
-
Catharanthine C16 substituent effects on the biomimetic coupling with vindoline: preparation and evaluation of a key series of vinblastine analogues.Bioorg Med Chem Lett. 2010 Nov 15;20(22):6408-10. doi: 10.1016/j.bmcl.2010.09.091. Epub 2010 Sep 19. Bioorg Med Chem Lett. 2010. PMID: 20932748 Free PMC article.
References
-
- Kutney JP, Chan KK, Failli A, Fromson JM, Gletsos C, Leutwiler A, Nelson VR. J Am Chem Soc. 1968;90:3891–3893. - PubMed
- Ziegler FE, Spitzner EB. J Am Chem Soc. 1973;95:7146–7149. - PubMed
- Kutney JP, Chan KK, Failli A, Fromson JM, Gletsos C, Leutwiler A, Nelson VR, de Souza JP. Helv Chim Acta. 1975;58:1648–1671. - PubMed
- Kuehne ME, Huebner JA, Matsko TH. J Org Chem. 1979;44:2477–2480.
- Kalaus G, Kiss M, Kajtar–Peredy M, Brlik J, Szabo L, Szantay C. Heterocycles. 1985;23:2783–2787.
-
-
Vasiliev NV, Lyashenko YE, Kolomiets AF, Sokolskii GA. Khim Geterotsikl Soedin. 1987:562.Vasiliev NV, Lyashenko YE, Galakhov MV, Kolomiets AF, Gontar AF, Sokolskii GA. Khim Geterotsikl Soedin. 1990:95–100.Vasiliev NV, Lyashenko YE, Patalakha AE, Sokolskii GA. J Fluorine Chem. 1993;65:227–231.Thalhammer F, Wallfahrer U, Sauer J. Tetrahedron Lett. 1988;29:3231–3234.Seitz G, Gerninghaus CH. Pharmazie. 1994;49:102–106.Seitz G, Wassmuth H. Chem -Ztg. 1988;112:80–81. Review: Warrener RN. Eur J Org Chem. 2000:3363–3380.Warrener RN, Margetic D, Foley PJ, Butler DN, Winling A, Beales KA, Russell RA. Tetrahedron. 2001;57:571–582.Warrener RN, Wang S, Maksimovic L, Tepperman PM, Butler DN. Tetrahedron Lett. 1995;36:6141–6144.Warrener RN, Elsey GM, Russell RA, Tiekink ERT. Tetrahedron Lett. 1995;36:5275–5278.Warrener RN, Maksimovic L, Butler DN. J Chem Soc Chem Commun. 1994:1831–1888.Warrener RN, Butler DN, Liao WY, Pitt IG, Butler DN, Russell RA. Tetrahedron Lett. 1991;32:1885–1888.Warrener RN, Margetic D, Tiekink ERT, Russell RA. Synlett. 1997:196–198.
-
-
- Christl M, Lanzendoerfer U, Groetsch MM, Ditterich E, Hegmann J. Chem Ber. 1990;123:2031–2037.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous