Plant ribulosamine/erythrulosamine 3-kinase, a putative protein-repair enzyme
- PMID: 15705060
- PMCID: PMC1183458
- DOI: 10.1042/BJ20041976
Plant ribulosamine/erythrulosamine 3-kinase, a putative protein-repair enzyme
Abstract
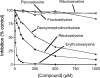
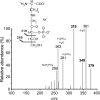
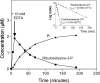
FN3K (fructosamine 3-kinase) is a mammalian enzyme that catalyses the phosphorylation of fructosamines, which thereby becomes unstable and detaches from proteins. The homologous mammalian enzyme, FN3K-RP (FN3K-related protein), does not phosphorylate fructosamines but ribulosamines, which are probably formed through a spontaneous reaction of amines with ribose 5-phosphate, an intermediate of the pentose-phosphate pathway and the Calvin cycle. We show in the present study that spinach leaf extracts display a substantial ribulosamine kinase activity (approx. 700 times higher than the specific activity of FN3K in erythrocytes). The ribulosamine kinase was purified approx. 400 times and shown to phosphorylate ribulose-epsilon-lysine, protein-bound ribulosamines and also, with higher affinity, erythrulose-epsilon-lysine and protein-bound erythrulosamines. Evidence is presented for the fact that the third carbon of the sugar portion is phosphorylated by this enzyme and that this leads to the formation of unstable compounds decomposing with half-lives of approx. 30 min at 37 degrees C (ribulosamine 3-phosphates) and 5 min at 30 degrees C (erythrulosamine 3-phosphates). This decomposition results in the formation of a 2-oxo-3-deoxyaldose and inorganic phosphate, with regeneration of the free amino group. The Arabidopsis thaliana homologue of FN3K/FN3K-RP was overexpressed in Escherichia coli and shown to have properties similar to those of the enzyme purified from spinach leaves. These results indicate that the plant FN3K/FN3K-RP homologue, which appears to be targeted to the chloroplast in many species, is a ribulosamine/erythrulosamine 3-kinase. This enzyme may participate in a protein deglycation process removing Amadori products derived from ribose 5-phosphate and erythrose 4-phosphate, two Calvin cycle intermediates that are potent glycating agents.
Figures




Similar articles
-
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially involved in protein deglycation.FEBS J. 2007 Sep;274(17):4360-74. doi: 10.1111/j.1742-4658.2007.05948.x. Epub 2007 Aug 6. FEBS J. 2007. PMID: 17681011
-
Enzymatic repair of Amadori products.Amino Acids. 2012 Apr;42(4):1143-50. doi: 10.1007/s00726-010-0780-3. Epub 2010 Oct 22. Amino Acids. 2012. PMID: 20967558 Review.
-
Fructosamine 3-kinase-related protein and deglycation in human erythrocytes.Biochem J. 2004 Aug 15;382(Pt 1):137-43. doi: 10.1042/BJ20040307. Biochem J. 2004. PMID: 15137908 Free PMC article.
-
A mammalian protein homologous to fructosamine-3-kinase is a ketosamine-3-kinase acting on psicosamines and ribulosamines but not on fructosamines.Diabetes. 2003 Dec;52(12):2888-95. doi: 10.2337/diabetes.52.12.2888. Diabetes. 2003. PMID: 14633848
-
Enzymatic deglycation--a new paradigm or an epiphenomenon?Biochem Soc Trans. 2003 Dec;31(Pt 6):1428-32. doi: 10.1042/bst0311428. Biochem Soc Trans. 2003. PMID: 14641081 Review.
Cited by
-
Identification of protein-ribulosamine-5-phosphatase as human low-molecular-mass protein tyrosine phosphatase-A.Biochem J. 2007 Aug 15;406(1):139-45. doi: 10.1042/BJ20061485. Biochem J. 2007. PMID: 17472574 Free PMC article.
-
Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling?Int J Mol Sci. 2019 May 13;20(9):2366. doi: 10.3390/ijms20092366. Int J Mol Sci. 2019. PMID: 31086058 Free PMC article. Review.
-
Multi-omics reveals new links between Fructosamine-3-Kinase (FN3K) and core metabolic pathways.NPJ Syst Biol Appl. 2024 Jun 3;10(1):64. doi: 10.1038/s41540-024-00390-0. NPJ Syst Biol Appl. 2024. PMID: 38830903 Free PMC article.
-
Structural and functional diversity of the microbial kinome.PLoS Biol. 2007 Mar;5(3):e17. doi: 10.1371/journal.pbio.0050017. PLoS Biol. 2007. PMID: 17355172 Free PMC article.
-
Protein Glycation in Plants-An Under-Researched Field with Much Still to Discover.Int J Mol Sci. 2020 May 30;21(11):3942. doi: 10.3390/ijms21113942. Int J Mol Sci. 2020. PMID: 32486308 Free PMC article. Review.
References
-
- Delpierre G., Rider M. H., Collard F., Stroobant V., Vanstapel F., Santos H., Van Schaftingen E. Identification, cloning, and heterologous expression of a mammalian fructosamine-3-kinase. Diabetes. 2000;49:1627–1634. - PubMed
-
- Szwergold B. S., Howell S., Beisswenger P. J. Human fructosamine-3-kinase: purification, sequencing, substrate specificity, and evidence of activity in vivo. Diabetes. 2001;50:2139–2147. - PubMed
-
- Hodge J. E. The Amadori rearrangement. Adv. Carbohyd. Chem. 1955;10:169–205. - PubMed
-
- Baynes J. W., Watkins N. G., Fisher C. I., Hull C. J., Patrick J. S., Ahmed M. U., Dunn J. A., Thorpe S. R. The Amadori product on protein: structure and reactions. Prog. Clin. Biol. Res. 1989;304:43–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

