Integration of the extranuclear and nuclear actions of estrogen
- PMID: 15705661
- PMCID: PMC1249516
- DOI: 10.1210/me.2004-0390
Integration of the extranuclear and nuclear actions of estrogen
Abstract
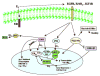
Estrogen receptors (ERs) are localized to many sites within the cell, potentially contributing to overall estrogen action. In the nucleus, estrogen mainly modulates gene transcription, and the resulting protein products determine the cell biological actions of the sex steroid. In addition, a small pool of ERs localize to the plasma membrane and signal mainly though coupling, directly or indirectly, to G proteins. In response to steroid, signal transduction modulates both nontranscriptional and transcriptional events and impacts both the rapid and more prolonged actions of estrogen. Cross-talk from membrane-localized ERs to nuclear ERs can be mediated through growth factor receptor tyrosine kinases, such as epidermal growth factor receptor and IGF-I receptor. Growth factor receptors enact signal transduction to kinases such as ERK and phosphatidylinositol 3-kinase that phosphorylate and activate nuclear ERs, and this can also occur in the absence of sex steroid. A complex relationship between the membrane and nuclear effects of estrogen also involves membrane-initiated phosphorylation of coactivators, recruiting these proteins to the nuclear transcriptosome. Finally, large pools of cytoplasmic ERs exist, and some are localized to mitochondria. The integration of sex steroid effects at distinct cellular locations of its receptor leads to important cellular physiological outcomes and are manifest in both reproductive and nonreproductive organs.
Figures
References
-
- Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Recent Prog Horm Res. 1962;18:387–414.
-
- Levin ER. Cell Localization, physiology and non-genomic actions of estrogen receptors. J Applied Physiol. 2001;91:1860–1867. - PubMed
-
- Kushner PJ, Agard D, Feng WJ, Lopez G, Schiau A, Uht R, Webb P, Greene G. Oestrogen receptor function at classical and alternative response elements. Novartis Found Symp. 2000;230:20–26. - PubMed
-
- Mandava MB. Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:23–52.
-
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRl1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

