Discussing randomised clinical trials of cancer therapy: evaluation of a Cancer Research UK training programme
- PMID: 15705666
- PMCID: PMC549112
- DOI: 10.1136/bmj.38366.562685.8F
Discussing randomised clinical trials of cancer therapy: evaluation of a Cancer Research UK training programme
Abstract
Objective: To evaluate a training intervention aimed at improving healthcare professionals' communication with cancer patients about randomised clinical trials.
Design: Before and after evaluation of training programme.
Setting: Members of the National Cancer Research Network, Scottish Trials Network, and the Welsh Cancer Trials Network.
Participants: 101 healthcare professionals (33 clinicians and 68 research nurses).
Intervention: Four modules delivered by a trained facilitator using videotapes and interactive exercises to cover general issues about discussing randomised clinical trials with patients, problems specific to adjuvant trials, trials with palliation as the goal, and trials where patients had a strong preference for one treatment arm.
Main outcome measures: Before and after the intervention, participants were videotaped discussing a trial with an actor portraying a patient. These consultations were assessed for presence of information required by good clinical practice guidelines. The actor patients gave an assessment after each interview. Participants reported their self confidence about key aspects of trial discussion.
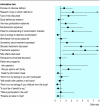
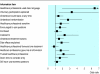
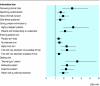
Results: Analysis of the videotaped consultations showed that, after intervention, significantly more participants displayed key communication behaviours such as explaining randomisation (69 v 81, odds ratio 2.33, P = 0.033), checking patients' understanding (11 v 31, odds ratio 3.22, P = 0.002), and discussing standard treatment (73 v 88, odds ratio 4.75, P = 0.005) and side effects (69 v 85, odds ratio 3.29, P = 0.006). Participants' self confidence increased significantly (P < 0.001) across all areas. Actor patients' ratings of participants' communication showed significant improvements for 12/15 key items.
Conclusion: This intensive 8 hour intervention significantly improved participants' confidence and competence when communicating about randomised clinical trials.
Figures

Similar articles
-
Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled trial.Lancet. 2002 Feb 23;359(9307):650-6. doi: 10.1016/S0140-6736(02)07810-8. Lancet. 2002. PMID: 11879860 Clinical Trial.
-
Improving general practitioners' interviewing skills in managing patients with depression and anxiety: a randomized controlled clinical trial.Med Teach. 2007 Sep;29(6):e175-83. doi: 10.1080/01421590601050585. Med Teach. 2007. PMID: 17917992 Clinical Trial.
-
Managing medical emergencies in mental health settings using an interprofessional in-situ simulation training programme: A mixed methods evaluation study.Nurse Educ Today. 2017 Dec;59:103-109. doi: 10.1016/j.nedt.2017.09.009. Epub 2017 Sep 21. Nurse Educ Today. 2017. PMID: 28968516
-
Effective communication skills are the key to good cancer care.Eur J Cancer. 1999 Oct;35(11):1592-7. doi: 10.1016/s0959-8049(99)00212-9. Eur J Cancer. 1999. PMID: 10673967 Review.
-
Improving communication with cancer patients.Eur J Cancer. 1999 Oct;35(10):1415-22. doi: 10.1016/s0959-8049(99)00178-1. Eur J Cancer. 1999. PMID: 10673972 Review.
Cited by
-
For the Benefit of Others: Reasons Why Women with Breast Cancer Participate in RCTs.Breast Care (Basel). 2015 Apr;10(2):88-93. doi: 10.1159/000376563. Breast Care (Basel). 2015. PMID: 26195936 Free PMC article.
-
Communication interventions in adult and pediatric oncology: A scoping review and analysis of behavioral targets.PLoS One. 2019 Aug 22;14(8):e0221536. doi: 10.1371/journal.pone.0221536. eCollection 2019. PLoS One. 2019. PMID: 31437262 Free PMC article.
-
The preferences of 600 patients for different descriptions of randomisation.Br J Cancer. 2005 Mar 14;92(5):807-10. doi: 10.1038/sj.bjc.6602445. Br J Cancer. 2005. PMID: 15726098 Free PMC article.
-
Ability of family members to predict patient's consent to critical care research.Intensive Care Med. 2007 May;33(5):807-813. doi: 10.1007/s00134-007-0582-6. Epub 2007 Mar 15. Intensive Care Med. 2007. PMID: 17361388
-
Communication about children's clinical trials as observed and experienced: qualitative study of parents and practitioners.PLoS One. 2011;6(7):e21604. doi: 10.1371/journal.pone.0021604. Epub 2011 Jul 12. PLoS One. 2011. PMID: 21765898 Free PMC article.
References
-
- Department of Health. Government response to the sixth report of the House of Commons Science and Technology Committee: session 1999/2000 cancer research—a fresh look. London: HMSO, 2000. (Cm 4928).
-
- Medical Research Council. MRC guidelines for good clinical practice in clinical trials. London: MRC, 1998.
-
- Albrecht TL, Blanchard C, Ruckdeschel JC, Coovert M, Strongbow R. Strategic physician communication and oncology clinical trials. J Clin Oncol 1999;17: 3324-32. - PubMed
-
- Fallowfield L, Saul J, Gilligan B. Teaching senior nurses how to teach communication skills in oncology. Cancer Nurs 2001;24: 185-91. - PubMed
-
- Fallowfield LJ, Jenkins VA. Effective communication skills are the key to good cancer care. Eur J Cancer 1999;35: 1592-7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
