Rectal artemether versus intravenous quinine for the treatment of cerebral malaria in children in Uganda: randomised clinical trial
- PMID: 15705690
- PMCID: PMC548725
- DOI: 10.1136/bmj.330.7487.334
Rectal artemether versus intravenous quinine for the treatment of cerebral malaria in children in Uganda: randomised clinical trial
Abstract
Objective: To compare the efficacy and safety of rectal artemether with intravenous quinine in the treatment of cerebral malaria in children.
Design: Randomised, single blind, clinical trial.
Setting: Acute care unit at Mulago Hospital, Uganda's national referral and teaching hospital in Kampala.
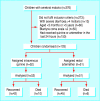
Participants: 103 children aged 6 months to 5 years with cerebral malaria.
Intervention: Patients were randomised to either intravenous quinine or rectal artemether for seven days.
Main outcome measures: Time to clearance of parasites and fever; time to regaining consciousness, starting oral intake, and sitting unaided; and adverse effects.
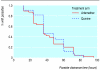
Results: The difference in parasitological and clinical outcomes between rectal artemether and intravenous quinine did not reach significance (parasite clearance time 54.2 (SD 33.6) hours v 55.0 (SD 24.3) hours, P = 0.90; fever clearance time 33.2 (SD 21.9) hours v 24.1(SD 18.9 hours, P = 0.08; time to regaining consciousness 30.1 (SD 24.1) hours v 22.67 (SD 18.5) hours, P = 0.10; time to starting oral intake 37.9 (SD 27.0) hours v 30.3 (SD 21.1) hours, P = 0.14). Mortality was higher in the quinine group than in the artemether group (10/52 v 6/51; relative risk 1.29, 95% confidence interval 0.84 to 2.01). No serious immediate adverse effects occurred.
Conclusion: Rectal artemether is effective and well tolerated and could be used as treatment for cerebral malaria.
Figures
Comment in
-
Treating severe malaria.BMJ. 2005 Feb 12;330(7487):317-8. doi: 10.1136/bmj.330.7487.317. BMJ. 2005. PMID: 15705667 Free PMC article. No abstract available.
Similar articles
-
Rectal versus intravenous quinine for the treatment of childhood cerebral malaria in Kampala, Uganda: a randomized, double-blind clinical trial.Clin Infect Dis. 2007 Dec 1;45(11):1446-52. doi: 10.1086/522972. Epub 2007 Oct 22. Clin Infect Dis. 2007. PMID: 17990227 Clinical Trial.
-
Comparison of intramuscular artemether and intravenous quinine in the treatment of Sudanese children with severe falciparum malaria.East Afr Med J. 2002 Dec;79(12):621-5. doi: 10.4314/eamj.v79i12.8668. East Afr Med J. 2002. PMID: 12683344 Clinical Trial.
-
Rectal dihydroartemisinin versus intravenous quinine in the treatment of severe malaria: a randomised clinical trial.East Afr Med J. 2000 May;77(5):273-8. doi: 10.4314/eamj.v77i5.46632. East Afr Med J. 2000. PMID: 12858920 Clinical Trial.
-
Artemether for severe malaria.Cochrane Database Syst Rev. 2019 Jun 18;6(6):CD010678. doi: 10.1002/14651858.CD010678.pub3. Cochrane Database Syst Rev. 2019. PMID: 31210357 Free PMC article.
-
Rectal artemisinins for malaria: a review of efficacy and safety from individual patient data in clinical studies.BMC Infect Dis. 2008 Mar 28;8:39. doi: 10.1186/1471-2334-8-39. BMC Infect Dis. 2008. PMID: 18373841 Free PMC article. Review.
Cited by
-
Pre-referral rectal artesunate for severe malaria.Cochrane Database Syst Rev. 2014 May 29;2014(5):CD009964. doi: 10.1002/14651858.CD009964.pub2. Cochrane Database Syst Rev. 2014. PMID: 24869943 Free PMC article.
-
Fever Is Associated with Reduced, Hypothermia with Increased Mortality in Septic Patients: A Meta-Analysis of Clinical Trials.PLoS One. 2017 Jan 12;12(1):e0170152. doi: 10.1371/journal.pone.0170152. eCollection 2017. PLoS One. 2017. PMID: 28081244 Free PMC article.
-
Clinical features and outcome in children with severe Plasmodium falciparum malaria: a meta-analysis.PLoS One. 2014 Feb 6;9(2):e86737. doi: 10.1371/journal.pone.0086737. eCollection 2014. PLoS One. 2014. PMID: 24516538 Free PMC article.
-
Malaria: severe, life-threatening.BMJ Clin Evid. 2007 Jul 1;2007:0913. BMJ Clin Evid. 2007. PMID: 19454095 Free PMC article.
-
Human immunodeficiency virus infection and cerebral malaria in children in Uganda: a case-control study.BMC Pediatr. 2011 Jan 14;11:5. doi: 10.1186/1471-2431-11-5. BMC Pediatr. 2011. PMID: 21235797 Free PMC article.
References
-
- Warrell D, ME M, Beales P. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84: 1-65. - PubMed
-
- Birku Y, Makonnen E, Bjorkman A. Comparison of rectal artemesinin with intravenous quinine in the treatment of severe malaria in Ethiopia. East Afr Med J 1999;76: 154-9. - PubMed
-
- Hien T, Arnold K, Vinh H, Cuong B, Phu N, Chau T, et al. Comparison of artemesinin suppositories with intravenous artesunate and intravenous quinine in the treatment of cerebral malaria. Trans R Soc Trop Med Hyg 1992;86: 582-3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical