Mouse Na+/K+-ATPase beta1-subunit has a K+-dependent cell adhesion activity for beta-GlcNAc-terminating glycans
- PMID: 15705719
- PMCID: PMC549466
- DOI: 10.1073/pnas.0409344102
Mouse Na+/K+-ATPase beta1-subunit has a K+-dependent cell adhesion activity for beta-GlcNAc-terminating glycans
Abstract
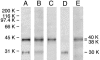
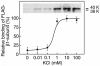
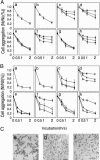
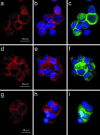
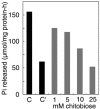
A 48-kDa beta-N-acetylglucosamine (GlcNAc)-binding protein was isolated from mouse brain by GlcNAc-agarose column chromatography. The N-terminal amino acid residues showed the protein to be a mouse Na(+)/K(+)-ATPase beta1-subunit. When the recombinant FLAG-beta1-subunit expressed in Sf-9 cells was applied to a GlcNAc-agarose column, only the glycosylated 38- and 40-kDa proteins bound to the column. In the absence of KCl, little of the proteins bound to a GlcNAc-agarose column, but the 38- and 40-kDa proteins bound in the presence of KCl at concentrations above 1 mM. Immunohistochemical study showed that the beta1-subunit and GlcNAc-terminating oligosaccharides are at the cell contact sites. Inclusion of anti-beta1-subunit antibody or chitobiose in cell aggregation assays using mouse neural cells resulted in inhibition of cell aggregation. These results indicate that the Na(+)/K(+)-ATPase beta1-subunit is a potassium-dependent lectin that binds to GlcNAc-terminating oligosaccharides: it may be involved in neural cell interactions.
Figures

Similar articles
-
The O-glycosylated ectodomain of FXYD5 impairs adhesion by disrupting cell-cell trans-dimerization of Na,K-ATPase β1 subunits.J Cell Sci. 2016 Jun 15;129(12):2394-406. doi: 10.1242/jcs.186148. Epub 2016 May 3. J Cell Sci. 2016. PMID: 27142834 Free PMC article.
-
Polarized membrane distribution of potassium-dependent ion pumps in epithelial cells: different roles of the N-glycans of their beta subunits.Cell Biochem Biophys. 2007;47(3):376-91. doi: 10.1007/s12013-007-0033-6. Cell Biochem Biophys. 2007. PMID: 17652782 Review.
-
Ouabain Enhances Cell-Cell Adhesion Mediated by β1 Subunits of the Na+,K+-ATPase in CHO Fibroblasts.Int J Mol Sci. 2019 Apr 29;20(9):2111. doi: 10.3390/ijms20092111. Int J Mol Sci. 2019. PMID: 31035668 Free PMC article.
-
Recombinant addition of N-glycosylation sites to the basolateral Na,K-ATPase beta1 subunit results in its clustering in caveolae and apical sorting in HGT-1 cells.J Biol Chem. 2005 Dec 30;280(52):43159-67. doi: 10.1074/jbc.M508262200. Epub 2005 Oct 17. J Biol Chem. 2005. PMID: 16230337
-
The roles of the Na,K-ATPase beta 1 subunit in pump sorting and epithelial integrity.J Bioenerg Biomembr. 2007 Dec;39(5-6):367-72. doi: 10.1007/s10863-007-9103-0. J Bioenerg Biomembr. 2007. PMID: 18000747 Review.
Cited by
-
β subunit affects Na+ and K+ affinities of Na+/K+-ATPase: Na+ and K+ affinities of a hybrid Na+/K+-ATPase composed of insect α and mammalian β subunits.Biochem Biophys Rep. 2022 Sep 14;32:101347. doi: 10.1016/j.bbrep.2022.101347. eCollection 2022 Dec. Biochem Biophys Rep. 2022. PMID: 36131851 Free PMC article.
-
Involvement of Galectin-3 with vascular cell adhesion molecule-1 in growth regulation of mouse BALB/3T3 cells.J Biol Chem. 2009 Dec 18;284(51):35556-63. doi: 10.1074/jbc.M109.063339. J Biol Chem. 2009. PMID: 19858221 Free PMC article.
-
Transcriptional regulators of Na,K-ATPase subunits.Front Cell Dev Biol. 2015 Oct 26;3:66. doi: 10.3389/fcell.2015.00066. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26579519 Free PMC article. Review.
-
Ion dependence of Na-K-ATPase-mediated epithelial cell adhesion and migration.Am J Physiol Cell Physiol. 2015 Sep 15;309(6):C437-41. doi: 10.1152/ajpcell.00140.2015. Epub 2015 Jul 8. Am J Physiol Cell Physiol. 2015. PMID: 26157008 Free PMC article. No abstract available.
-
Sodium-calcium exchanger 1 regulates epithelial cell migration via calcium-dependent extracellular signal-regulated kinase signaling.J Biol Chem. 2015 May 15;290(20):12463-73. doi: 10.1074/jbc.M114.629519. Epub 2015 Mar 13. J Biol Chem. 2015. PMID: 25770213 Free PMC article.
References
-
- Furukawa, K., Takamiya, K., Okada, M., Inoue, M., Fukumoto, S. & Furukawa, K. (2001) Biochim. Biophys. Acta 1525, 1–12. - PubMed
-
- Helenius, A. & Aebi, M. (2004) Annu. Rev. Biochem. 73, 1019–1049. - PubMed
-
- Chen, Y.-J., Wing, D. R., Guile, G. R., Dwek, R. A., Harvey, D. J. & Zamze, S. (1998) Eur. J. Biochem. 251, 691–703. - PubMed
-
- Shimizu, H., Ochi, K., Ikenaka, K., Mikoshiba, K. & Hase, S. (1993) J. Biochem. (Tokyo) 114, 334–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

