Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways
- PMID: 15705783
- PMCID: PMC1894992
- DOI: 10.1182/blood-2004-10-4093
Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways
Abstract
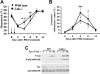
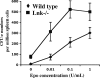
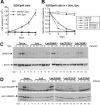
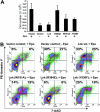
Erythropoietin (Epo), along with its receptor EpoR, is the principal regulator of red cell development. Upon Epo addition, the EpoR signaling through the Janus kinase 2 (JAK2) activates multiple pathways including Stat5, phosphoinositide-3 kinase (PI-3K)/Akt, and p42/44 mitogen-activated protein kinase (MAPK). The adaptor protein Lnk is implicated in cytokine receptor signaling. Here, we showed that Lnk-deficient mice have elevated numbers of erythroid progenitors, and that splenic erythroid colony-forming unit (CFU-e) progenitors are hypersensitive to Epo. Lnk(-/-) mice also exhibit superior recovery after erythropoietic stress. In addition, Lnk deficiency resulted in enhanced Epo-induced signaling pathways in splenic erythroid progenitors. Conversely, Lnk overexpression inhibits Epo-induced cell growth in 32D/EpoR cells. In primary culture of fetal liver cells, Lnk overexpression inhibits Epo-dependent erythroblast differentiation and induces apoptosis. Lnk blocks 3 major signaling pathways, Stat5, Akt, and MAPK, induced by Epo in primary erythroblasts. In addition, the Lnk Src homology 2 (SH2) domain is essential for its inhibitory function, whereas the conserved tyrosine near the C-terminus and the pleckstrin homology (PH) domain of Lnk are not critical. Furthermore, wild-type Lnk, but not the Lnk SH2 mutant, becomes tyrosine-phosphorylated following Epo administration and inhibits EpoR phosphorylation and JAK2 activation. Hence, Lnk, through its SH2 domain, negatively modulates EpoR signaling by attenuating JAK2 activation, and regulates Epo-mediated erythropoiesis.
Figures



Similar articles
-
Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis.J Exp Med. 2004 Sep 6;200(5):569-80. doi: 10.1084/jem.20040762. Epub 2004 Aug 30. J Exp Med. 2004. PMID: 15337790 Free PMC article.
-
A novel role for STAT1 in regulating murine erythropoiesis: deletion of STAT1 results in overall reduction of erythroid progenitors and alters their distribution.Blood. 2005 Jan 15;105(2):552-61. doi: 10.1182/blood-2003-09-3237. Epub 2004 Jun 22. Blood. 2005. PMID: 15213094
-
Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway.Blood. 1998 May 15;91(10):3734-45. Blood. 1998. PMID: 9573010
-
Control of erythropoiesis by erythropoietin and stem cell factor: a novel role for Bruton's tyrosine kinase.Cell Cycle. 2004 Jul;3(7):876-9. Epub 2004 Jul 2. Cell Cycle. 2004. PMID: 15254422 Review.
-
STAT5 as a Key Protein of Erythropoietin Signalization.Int J Mol Sci. 2021 Jul 1;22(13):7109. doi: 10.3390/ijms22137109. Int J Mol Sci. 2021. PMID: 34281163 Free PMC article. Review.
Cited by
-
Influence of Genetic Variants in EGF and Other Genes on Hematological Traits in Korean Populations by a Genome-Wide Approach.Biomed Res Int. 2015;2015:914965. doi: 10.1155/2015/914965. Epub 2015 Apr 30. Biomed Res Int. 2015. PMID: 26064965 Free PMC article.
-
SH2B3 (LNK) mutations from myeloproliferative neoplasms patients have mild loss of function against wild type JAK2 and JAK2 V617F.Br J Haematol. 2013 Jun;161(6):811-20. doi: 10.1111/bjh.12327. Epub 2013 Apr 17. Br J Haematol. 2013. PMID: 23590807 Free PMC article.
-
A nonsynonymous LNK polymorphism associated with idiopathic erythrocytosis.Am J Hematol. 2011 Nov;86(11):962-4. doi: 10.1002/ajh.22154. Epub 2011 Aug 22. Am J Hematol. 2011. PMID: 21990094 Free PMC article.
-
JAK2 mutants (e.g., JAK2V617F) and their importance as drug targets in myeloproliferative neoplasms.JAKSTAT. 2013 Jul 1;2(3):e25025. doi: 10.4161/jkst.25025. Epub 2013 May 14. JAKSTAT. 2013. PMID: 24069563 Free PMC article. Review.
-
The molecular regulation of Janus kinase (JAK) activation.Biochem J. 2014 Aug 15;462(1):1-13. doi: 10.1042/BJ20140712. Biochem J. 2014. PMID: 25057888 Free PMC article. Review.
References
-
- Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83: 59-67. - PubMed
-
- Socolovsky M, Constantinescu SN, Bergelson S, Sirotkin A, Lodish HF. Cytokines in hematopoiesis: specificity and redundancy in receptor function. Adv Protein Chem. 1998;52: 141-198. - PubMed
-
- Wojchowski DM, Gregory RC, Miller CP, Pandit AK, Pircher TJ. Signal transduction in the erythropoietin receptor system. Exp Cell Res. 1999;253: 143-156. - PubMed
-
- Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93: 385-395. - PubMed
-
- Neubauer H, Cumano A, Muller M, Wu H, Huff-stadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93: 397-409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

