Azaspirane (N-N-diethyl-8,8-dipropyl-2-azaspiro [4.5] decane-2-propanamine) inhibits human multiple myeloma cell growth in the bone marrow milieu in vitro and in vivo
- PMID: 15705788
- PMCID: PMC1895034
- DOI: 10.1182/blood-2004-09-3794
Azaspirane (N-N-diethyl-8,8-dipropyl-2-azaspiro [4.5] decane-2-propanamine) inhibits human multiple myeloma cell growth in the bone marrow milieu in vitro and in vivo
Abstract
Azaspirane (N-N-diethyl-8,8-dipropyl-2-azaspiro [4.5] decane-2-propanamine; trade name, Atiprimod) is an orally bioavailable cationic amphiphilic compound that significantly inhibits production of interleukin 6 (IL-6) and inflammation in rat arthritis and autoimmune animal models. We here characterize the effect of atiprimod on human multiple myeloma (MM) cells. Azaspirane significantly inhibited growth and induced caspase-mediated apoptosis in drug-sensitive and drug-resistant MM cell lines, as well as patient MM cells. IL-6, insulin-like growth factor 1 (IGF-1), or adherence of MM cells to bone marrow stromal cells (BMSCs) did not protect against atiprimod-induced apoptosis. Both conventional (dexamethasone, doxorubicin, melphalan) and novel (arsenic trioxide) agents augment apoptosis induced by atiprimod. Azaspirane inhibits signal transducer activator of transcription 3 (STAT3) and a PI3-K (phosphatidylinositol 3-kinase) target (Akt), but not extracellular signal-regulated kinase 1 and 2 (ERK1/2), inhibits phosphorylation triggered by IL-6, and also inhibits inhibitorkappaBalpha (IkappaBalpha) and nuclear factor kappaB (NFkappaB) p65 phosphorylation triggered by tumor necrosis factor alpha (TNF-alpha). Of importance, azaspirane inhibits both IL-6 and vascular endothelial growth factor (VEGF) secretion in BMSCs triggered by MM cell binding and also inhibits angiogenesis on human umbilical vein cells (HUVECs). Finally, azaspirane demonstrates in vivo antitumor activity against human MM cell growth in severe combined immunodeficient (SCID) mice. These results, therefore, show that azaspirane both induces MM cell apoptosis and inhibits cytokine secretion in the BM milieu, providing the framework for clinical trials to improve patient outcome in MM.
Figures
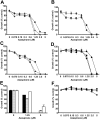
 , □), as well as (F) peripheral blood mononuclear cells from healthy volunteers (n = 3; ▪, ▴, ♦) were cultured for 48 hours in the presence of azaspirane (0-5 μM). Cell growth was assessed by MTT assay, and data represent mean (± SD) of quadruplicate cultures.
, □), as well as (F) peripheral blood mononuclear cells from healthy volunteers (n = 3; ▪, ▴, ♦) were cultured for 48 hours in the presence of azaspirane (0-5 μM). Cell growth was assessed by MTT assay, and data represent mean (± SD) of quadruplicate cultures.
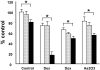
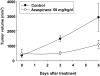
 ) or 1.25 μM (▪) azaspirane in the absence or presence of Dex (0.5 μM), Dox (0.5 μM), and As2O3 (1 μM) for 24 hours. Cell growth was assessed by MTT assay, and data represent mean (± SD) of quadruplicate cultures. *P < .01.
) or 1.25 μM (▪) azaspirane in the absence or presence of Dex (0.5 μM), Dox (0.5 μM), and As2O3 (1 μM) for 24 hours. Cell growth was assessed by MTT assay, and data represent mean (± SD) of quadruplicate cultures. *P < .01.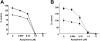

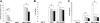
 ), 1.25 μM(
), 1.25 μM( ), and 2.5 μM(▪) azaspirane (A). DNA synthesis was assessed by [3H]-thymidine uptake, and data represent mean (± SD) of quadruplicate cultures. IL-6 (B) and VEGF (C) levels in culture supernatants were measured by ELISA. *P < .01.
), and 2.5 μM(▪) azaspirane (A). DNA synthesis was assessed by [3H]-thymidine uptake, and data represent mean (± SD) of quadruplicate cultures. IL-6 (B) and VEGF (C) levels in culture supernatants were measured by ELISA. *P < .01.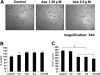
Similar articles
-
MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo.Clin Cancer Res. 2006 Oct 1;12(19):5887-94. doi: 10.1158/1078-0432.CCR-05-2501. Clin Cancer Res. 2006. PMID: 17020997
-
Novel therapies targeting the myeloma cell and its bone marrow microenvironment.Semin Oncol. 2001 Dec;28(6):607-12. doi: 10.1016/s0093-7754(01)90033-8. Semin Oncol. 2001. PMID: 11740818 Review.
-
Atiprimod is an inhibitor of cancer cell proliferation and angiogenesis.J Exp Ther Oncol. 2004 Dec;4(4):267-79. J Exp Ther Oncol. 2004. PMID: 15844657
-
Atiprimod blocks STAT3 phosphorylation and induces apoptosis in multiple myeloma cells.Br J Cancer. 2005 Jul 11;93(1):70-80. doi: 10.1038/sj.bjc.6602637. Br J Cancer. 2005. PMID: 15970928 Free PMC article.
-
Targeted therapies in multiple myeloma.Target Oncol. 2009 Jan;4(1):23-36. doi: 10.1007/s11523-008-0102-9. Epub 2009 Jan 17. Target Oncol. 2009. PMID: 19343299 Review.
Cited by
-
Mouse models of multiple myeloma: technologic platforms and perspectives.Oncotarget. 2018 Apr 13;9(28):20119-20133. doi: 10.18632/oncotarget.24614. eCollection 2018 Apr 13. Oncotarget. 2018. PMID: 29732008 Free PMC article. Review.
-
Blockade of interleukin-6 signalling with siltuximab enhances melphalan cytotoxicity in preclinical models of multiple myeloma.Br J Haematol. 2011 Mar;152(5):579-92. doi: 10.1111/j.1365-2141.2010.08533.x. Epub 2011 Jan 17. Br J Haematol. 2011. PMID: 21241278 Free PMC article.
-
A Triazaspirane Derivative Inhibits Migration and Invasion in PC3 Prostate Cancer Cells.Molecules. 2023 Jun 2;28(11):4524. doi: 10.3390/molecules28114524. Molecules. 2023. PMID: 37299000 Free PMC article.
-
Emerging therapies for multiple myeloma.Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. doi: 10.1517/14728210802676278. Expert Opin Emerg Drugs. 2009. PMID: 19249983 Free PMC article. Review.
-
Bone marrow microenvironment and the identification of new targets for myeloma therapy.Leukemia. 2009 Jan;23(1):10-24. doi: 10.1038/leu.2008.259. Epub 2008 Oct 9. Leukemia. 2009. PMID: 18843284 Free PMC article. Review.
References
-
- Gregory W, Richards M, Malpas J. Combination chemotherapy versus melphalan and prednisolone in the treatment of multiple myeloma: an overview of published trials. J Clin Oncol. 1992;10: 334-342. - PubMed
-
- Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists' Collaborative Group. J Clin Oncol. 1998;16: 3832-3842. - PubMed
-
- Fermand J, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment?. Results of a multicenter sequential randomized clinical trial. Blood. 1998;92: 3131-3136. - PubMed
-
- Lenhoff S, Hjorth M, Holmberg E, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood. 2000;95: 7-11. - PubMed
-
- Attal M, Harousseau J. Randomized trial experience of the Intergroupe Francophone du Myelome. Semin Hematol. 2001;38: 226-230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

