The relative role of PLCbeta and PI3Kgamma in platelet activation
- PMID: 15705797
- PMCID: PMC1895115
- DOI: 10.1182/blood-2004-05-2005
The relative role of PLCbeta and PI3Kgamma in platelet activation
Abstract
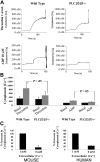
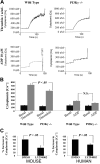
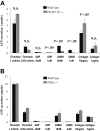
Stimulation of platelet G protein-coupled receptors results in the cleavage of phosphatidylinositol 4,5-trisphosphate (PIP(2)) into inositol 1,4,5-trisphosphate and 1,2-diacylglycerol by phospholipase C (PLCbeta). It also results in the phosphorylation of PIP2 by the gamma isoform of phosphatidylinositol 3-kinase (PI3Kgamma) to synthesize phosphatidylinositol 3,4,5-trisphosphate. To understand the role of PIP2 in platelet signaling, we evaluated knock-out mice lacking 2 isoforms of PLCbeta (PLCbeta2 and PLCbeta3) or lacking the G(betagamma)-activated isoform of PI3K (PI3Kgamma). Both knock-out mice were unable to form stable thrombi in a carotid injury model. To provide a functional explanation, knock-out platelets were studied ex vivo. PLCbeta2/beta3-/- platelets failed to assemble filamentous actin, had defects in both secretion and mobilization of intracellular calcium, and were unable to form stable aggregates following low doses of agonists. Platelets lacking PI3Kgamma disaggregated following low-dose adenosine diphosphate (ADP) and had a mildly impaired ability to mobilize intracellular calcium. Yet, they exhibited essentially normal actin assembly and secretion. Remarkably, both PLCbeta2/beta3-/- and PI3Kgamma-/- platelets spread more slowly upon fibrinogen. These results suggest substantial redundancy in platelet signaling pathways. Nonetheless, the diminished ability of knock-out platelets to normally spread after adhesion and to form stable thrombi in vivo suggests that both PLCbeta2/beta3 and PI3Kgamma play vital roles in platelet cytoskeletal dynamics.
Figures






References
-
- Rittenhouse SE. Phosphoinositide 3-kinase activation and platelet function. Blood. 1996;88: 4401-4414. - PubMed
-
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272: 15045-15048. - PubMed
-
- Lapetina EG, Reep B, Ganong BR, Bell RM. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985;260: 1358-1361. - PubMed
-
- Brass LF, Joseph SK. A role for inositol triphosphate in intracellular Ca2+ mobilization and granule secretion in platelets. J Biol Chem. 1985;260: 15172-15179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

