A vacuolar processing enzyme, deltaVPE, is involved in seed coat formation at the early stage of seed development
- PMID: 15705955
- PMCID: PMC1069705
- DOI: 10.1105/tpc.104.026872
A vacuolar processing enzyme, deltaVPE, is involved in seed coat formation at the early stage of seed development
Abstract
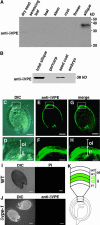
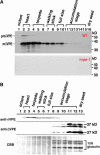
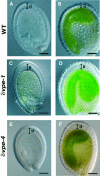
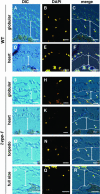
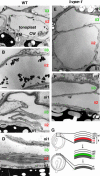
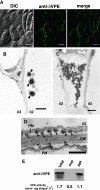
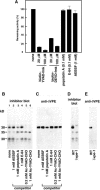
Vacuolar processing enzyme (VPE) is a Cys proteinase responsible for the maturation of vacuolar proteins. Arabidopsis thaliana deltaVPE, which was recently found in the database, was specifically and transiently expressed in two cell layers of the seed coat (ii2 and ii3) at an early stage of seed development. At this stage, cell death accompanying cell shrinkage occurs in the ii2 layer followed by cell death in the ii3 layer. In a deltaVPE-deficient mutant, cell death of the two layers of the seed coat was delayed. Immunocytochemical analysis localized deltaVPE to electron-dense structures inside and outside the walls of seed coat cells that undergo cell death. Interestingly, deltaVPE in the precipitate fraction from young siliques exhibits caspase-1-like activity, which has been detected in various types of plant cell death. Our results suggest that, at the early stage of seed development, deltaVPE is involved in cell death of limited cell layers, the purpose of which is to form a seed coat.
Figures

Similar articles
-
Storage protein accumulation in the absence of the vacuolar processing enzyme family of cysteine proteases.Plant Cell. 2004 Jan;16(1):270-90. doi: 10.1105/tpc.016378. Epub 2003 Dec 19. Plant Cell. 2004. PMID: 14688293 Free PMC article.
-
Redundant proteolytic mechanisms process seed storage proteins in the absence of seed-type members of the vacuolar processing enzyme family of cysteine proteases.Plant Cell. 2002 Nov;14(11):2863-82. doi: 10.1105/tpc.005009. Plant Cell. 2002. PMID: 12417707 Free PMC article.
-
Tissue-specific transcriptome analysis reveals cell wall metabolism, flavonol biosynthesis and defense responses are activated in the endosperm of germinating Arabidopsis thaliana seeds.Plant Cell Physiol. 2012 Jan;53(1):16-27. doi: 10.1093/pcp/pcr171. Epub 2011 Dec 5. Plant Cell Physiol. 2012. PMID: 22147073
-
Genetic analysis of seed coat development in Arabidopsis.Trends Plant Sci. 2005 Oct;10(10):472-7. doi: 10.1016/j.tplants.2005.08.005. Trends Plant Sci. 2005. PMID: 16153880 Review.
-
Vacuolar processing enzyme: an executor of plant cell death.Curr Opin Plant Biol. 2005 Aug;8(4):404-8. doi: 10.1016/j.pbi.2005.05.016. Curr Opin Plant Biol. 2005. PMID: 15939660 Review.
Cited by
-
Vacuolar Processing Enzymes in Plant Programmed Cell Death and Autophagy.Int J Mol Sci. 2023 Jan 7;24(2):1198. doi: 10.3390/ijms24021198. Int J Mol Sci. 2023. PMID: 36674706 Free PMC article. Review.
-
Expression of IbVPE1 from sweet potato in Arabidopsis affects leaf development, flowering time and chlorophyll catabolism.BMC Plant Biol. 2019 May 6;19(1):184. doi: 10.1186/s12870-019-1789-8. BMC Plant Biol. 2019. PMID: 31060496 Free PMC article.
-
Short-term waterlogging-induced autophagy in root cells of wheat can inhibit programmed cell death.Protoplasma. 2021 Jul;258(4):891-904. doi: 10.1007/s00709-021-01610-8. Epub 2021 Jan 23. Protoplasma. 2021. PMID: 33486619
-
Gene Cloning, Expression and Enzyme Activity of Vitis vinifera Vacuolar Processing Enzymes (VvVPEs).PLoS One. 2016 Aug 23;11(8):e0160945. doi: 10.1371/journal.pone.0160945. eCollection 2016. PLoS One. 2016. PMID: 27551866 Free PMC article.
-
The Amborella vacuolar processing enzyme family.Front Plant Sci. 2015 Aug 21;6:618. doi: 10.3389/fpls.2015.00618. eCollection 2015. Front Plant Sci. 2015. PMID: 26347753 Free PMC article.
References
-
- Asamizu, E., Nakamura, Y., Sato, S., and Tabata, S. (2000). A large scale analysis of cDNA in Arabidopsis thaliana: Generation of 12,028 non-redundant expressed sequence tags from normalized and size-selected cDNA libraries. DNA Res. 7, 175–180. - PubMed
-
- Becker, C., Shutov, A.D., Nong, V.H., Senyuk, V.I., Jung, R., Horstmann, C., Fischer, J., Nielsen, N.C., and Muntz, K. (1995). Purification, cDNA cloning and characterization of proteinase B, an asparagine-specific endopeptidase from germinating vetch (Vicia sativa L.) seeds. Eur. J. Biochem. 228, 456–462. - PubMed
-
- Beeckman, T., De Rycke, R., Viane, R., and Inze, D. (2000). Histological study of seed coat development in Arabidopsis thaliana. J. Plant Res. 113, 139–148.
-
- Caffrey, C.R., Mathieu, M.A., Gaffney, A.M., Salter, J.P., Sajid, M., Lucas, K.D., Franklin, C., Bogyo, M., and McKerrow, J.H. (2000). Identification of a cDNA encoding an active asparaginyl endopeptidase of Schistosoma mansoni and its expression in Pichia pastoris. FEBS Lett. 466, 244–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

