Redox mechanisms of cytoprotection by Bcl-2
- PMID: 15706098
- PMCID: PMC2570327
- DOI: 10.1089/ars.2005.7.508
Redox mechanisms of cytoprotection by Bcl-2
Abstract
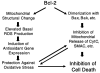
Bcl-2 is a multifunctional protein that protects against cell death induced by a wide variety of stimuli. The best characterized antiapoptotic Bcl-2 mechanism of action involves direct binding to proapoptotic proteins, e.g., Bax, inhibiting their ability to oligomerize and form pores in the mitochondrial outer membrane, through which soluble mitochondrial proapoptotic proteins, e.g., cytochrome c, are released into the cytosol. Bcl-2 also exerts antiapoptotic and antinecrotic effects that are mediated by its influence on cellular redox state and apparently independent of its interaction with proapoptotic proteins. Bcl-2 expression increases cell resistance to oxidants, augments the expression of intracellular defenses against reactive oxygen species, and may affect mitochondrial generation of superoxide radicals and hydrogen peroxide. This review focuses on the protective effects of Bcl-2 related to changes in mitochondrial redox capacity.
Figures

Similar articles
-
Bcl-2 family proteins regulate mitochondrial reactive oxygen production and protect against oxidative stress.Free Radic Biol Med. 2004 Dec 1;37(11):1845-53. doi: 10.1016/j.freeradbiomed.2004.09.005. Free Radic Biol Med. 2004. PMID: 15528043
-
Inhibition of mitochondrial neural cell death pathways by protein transduction of Bcl-2 family proteins.J Bioenerg Biomembr. 2005 Jun;37(3):179-90. doi: 10.1007/s10863-005-6590-8. J Bioenerg Biomembr. 2005. PMID: 16167175 Free PMC article. Review.
-
Mitochondrial genome depletion in human liver cells abolishes bile acid-induced apoptosis: role of the Akt/mTOR survival pathway and Bcl-2 family proteins.Free Radic Biol Med. 2013 Aug;61:218-28. doi: 10.1016/j.freeradbiomed.2013.04.002. Epub 2013 Apr 16. Free Radic Biol Med. 2013. PMID: 23597504
-
Idebenone Prevents Oxidative Stress, Cell Death and Senescence of Retinal Pigment Epithelium Cells by Stabilizing BAX/Bcl-2 Ratio.Ophthalmologica. 2015;234(2):73-82. doi: 10.1159/000381726. Epub 2015 Jun 4. Ophthalmologica. 2015. PMID: 26044821
-
Utilization of yeast to investigate the role of lipid oxidation in cell death.Antioxid Redox Signal. 2004 Apr;6(2):259-67. doi: 10.1089/152308604322899323. Antioxid Redox Signal. 2004. PMID: 15025927 Review.
Cited by
-
Inhibition of B-cell lymphoma 2 family proteins alters optical redox ratio, mitochondrial polarization, and cell energetics independent of cell state.J Biomed Opt. 2022 May;27(5):056505. doi: 10.1117/1.JBO.27.5.056505. J Biomed Opt. 2022. PMID: 35643815 Free PMC article.
-
Oxygen in human health from life to death--An approach to teaching redox biology and signaling to graduate and medical students.Redox Biol. 2015 Aug;5:124-139. doi: 10.1016/j.redox.2015.04.002. Epub 2015 Apr 11. Redox Biol. 2015. PMID: 25912168 Free PMC article. Review.
-
Rasagiline prevents apoptosis induced by PK11195, a ligand of the outer membrane translocator protein (18 kDa), in SH-SY5Y cells through suppression of cytochrome c release from mitochondria.J Neural Transm (Vienna). 2013 Nov;120(11):1539-51. doi: 10.1007/s00702-013-1033-x. Epub 2013 May 17. J Neural Transm (Vienna). 2013. PMID: 23681678
-
Protective effects of ascorbic acid on behavior and oxidative status of restraint-stressed mice.J Mol Neurosci. 2013 Jan;49(1):68-79. doi: 10.1007/s12031-012-9892-4. Epub 2012 Oct 3. J Mol Neurosci. 2013. PMID: 23054587
-
Calcium uptake and cytochrome c release from normal and ischemic brain mitochondria.Neurochem Int. 2018 Jul;117:15-22. doi: 10.1016/j.neuint.2017.10.003. Epub 2017 Oct 16. Neurochem Int. 2018. PMID: 29042253 Free PMC article.
References
-
- Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. - PubMed
-
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. - PubMed
-
- Cadenas E, Boveris A, Ragan CI, Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977;180:248–257. - PubMed
-
- Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

