Redox mechanisms of cytoprotection by Bcl-2
- PMID: 15706098
- PMCID: PMC2570327
- DOI: 10.1089/ars.2005.7.508
Redox mechanisms of cytoprotection by Bcl-2
Abstract
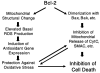
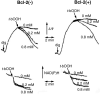
Bcl-2 is a multifunctional protein that protects against cell death induced by a wide variety of stimuli. The best characterized antiapoptotic Bcl-2 mechanism of action involves direct binding to proapoptotic proteins, e.g., Bax, inhibiting their ability to oligomerize and form pores in the mitochondrial outer membrane, through which soluble mitochondrial proapoptotic proteins, e.g., cytochrome c, are released into the cytosol. Bcl-2 also exerts antiapoptotic and antinecrotic effects that are mediated by its influence on cellular redox state and apparently independent of its interaction with proapoptotic proteins. Bcl-2 expression increases cell resistance to oxidants, augments the expression of intracellular defenses against reactive oxygen species, and may affect mitochondrial generation of superoxide radicals and hydrogen peroxide. This review focuses on the protective effects of Bcl-2 related to changes in mitochondrial redox capacity.
Figures

References
-
- Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. - PubMed
-
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. - PubMed
-
- Cadenas E, Boveris A, Ragan CI, Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977;180:248–257. - PubMed
-
- Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

