Disease-associated prion protein elicits immunoglobulin M responses in vivo
- PMID: 15706401
- PMCID: PMC1431372
- DOI: 10.2119/2004-00027.Tayebi
Disease-associated prion protein elicits immunoglobulin M responses in vivo
Abstract
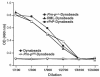
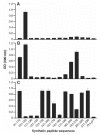
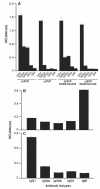
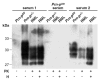
Prion diseases such as Creutzfeldt-Jakob disease are believed to result from the misfolding of a widely expressed normal cellular prion protein, PrPc. The resulting disease-associated isoforms, PrP(Sc), have much higher beta-sheet content, are insoluble in detergents, and acquire relative resistance to proteases. Although known to be highly aggregated and to form amyloid fibrils, the molecular architecture of PrP9Sc) is poorly understood. To date, it has been impossible to elicit antibodies to native PrP(Sc) that are capable of recognizing PrP(Sc) without denaturation, even in Pm-P(o/o) mice that are intolerant of it. Here we demonstrate that antibodies for native PrPc and PrP(Sc) can be produced by immunization of Pm-P(o/o) mice with partially purified PrPc and PrP(Sc) adsorbed to immunomagnetic particles using high-affinity anti-PrP monoclonal antibodies (mAbs). Interestingly, the polyclonal response to PrP(Sc) was predominantly of the immunoglobulin M (IgM) isotype, unlike the immunoglobulin G (IgG) responses elicited by PrP(c) or by recombinant PrP adsorbed or not to immunomagnetic particles, presumably reflecting the polymeric structure of disease-associated prion protein. Although heat-denatured PrP(Sc) elicited more diverse antibodies with the revelation of C-terminal epitopes, remarkably, these were also predominantly IgM suggesting that the increasing immunogenicity, acquisition of protease sensitivity, and reduction in infectivity induced by heat are not associated with dissociation of the PrP molecules in the diseased-associated protein. Adsorbing native proteins to immunomagnetic particles may have general applicability for raising polyclonal or monoclonal antibodies to any native protein, without attempting laborious purification steps that might affect protein conformation.
Figures




References
-
- Prusiner SB. Molecular biology and genetics of prion diseases. Philos Trans R Soc Lond B Biol Sci. 1994;343:447–63. - PubMed
-
- Gray F, et al. Creutzfeldt-Jakob disease and cerebral amyloid angiopathy. Acta Neuropathol (Berl) 1994;88:106–11. - PubMed
-
- Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. - PubMed
-
- Wells GAH, et al. A novel progressive spongiform encephalopathy in cattle. Vet Record Oct. 1987;31:419–20. - PubMed
-
- Wilesmith JW, Wells GA, Cranwell MP, Ryan JB. Bovine spongiform encephalopathy: epidemiological studies. Vet Record. 1988;123:638–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
