Mass spectrometrical analysis of recombinant human growth hormone (Genotropin(R)) reveals amino acid substitutions in 2% of the expressed protein
- PMID: 15707495
- PMCID: PMC549540
- DOI: 10.1186/1477-5956-3-1
Mass spectrometrical analysis of recombinant human growth hormone (Genotropin(R)) reveals amino acid substitutions in 2% of the expressed protein
Abstract
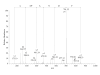
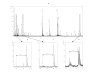
BACKGROUND: The structural integrity of recombinant proteins is of critical importance to their application as clinical treatments. Recombinant growth hormone preparations have been examined by several methodologies. In this study recombinant human growth hormone (rhGH; Genotropin(R)), expressed in E. coli K12, was structurally analyzed by two-dimensional gel electrophoresis and MALDI-TOF-TOF, LC-MS and LC-MS/ MS sequencing of the resolved peptides. RESULTS: Electrospray LC-MS analysis revealed one major protein with an average molecular mass of 22126.8 Da and some additional minor components. Electrospray LC-MS/MS evaluation of the enzymatically digested Genotropin(R) sample resulted in the identification of amino acid substitutions at the residues M14, M125, and M170; di-methylation of K70 (or exchange to arginine); deamidation of N149, and N152, and oxidation of M140, M125 and M170. Peak area comparison of the modified and parental peptides indicates that these changes were present in ~2% of the recombinant preparation. CONCLUSION: Modifications of the recombinant human growth hormone may lead to structural or conformational changes, modification of antigenicity and development of antibody formation in treated subjects. Amino acid exchanges may be caused by differences between human and E. coli codon usage and/or unknown copy editing mechanisms. While deamidation and oxidation can be assigned to processing events, the mechanism for possible di-methylation of K70 remains unclear.
Figures



Similar articles
-
Massspectrometrical analysis of recombinant human growth hormone Norditropin reveals amino acid exchange at M14_V14 rhGH.Proteomics. 2006 Feb;6(3):775-84. doi: 10.1002/pmic.200500334. Proteomics. 2006. PMID: 16372270
-
Identification of 2D-gel proteins: a comparison of MALDI/TOF peptide mass mapping to mu LC-ESI tandem mass spectrometry.J Am Soc Mass Spectrom. 2003 Sep;14(9):957-70. doi: 10.1016/s1044-0305(03)00144-2. J Am Soc Mass Spectrom. 2003. PMID: 12954164
-
Targeted proteomics of low-level proteins in human plasma by LC/MSn: using human growth hormone as a model system.J Proteome Res. 2002 Sep-Oct;1(5):459-65. doi: 10.1021/pr025537l. J Proteome Res. 2002. PMID: 12645918
-
Mass spectrometry and tandem mass spectrometry characterization of protein patterns, protein markers and whole proteomes for pathogenic bacteria.J Microbiol Methods. 2013 Mar;92(3):381-6. doi: 10.1016/j.mimet.2013.01.004. Epub 2013 Jan 12. J Microbiol Methods. 2013. PMID: 23318550 Review.
-
Detection of oxidized and glycated proteins in clinical samples using mass spectrometry--a user's perspective.Biochim Biophys Acta. 2014 Feb;1840(2):818-29. doi: 10.1016/j.bbagen.2013.03.025. Epub 2013 Apr 2. Biochim Biophys Acta. 2014. PMID: 23558060 Review.
Cited by
-
Oxidation of therapeutic proteins and peptides: structural and biological consequences.Pharm Res. 2014 Mar;31(3):541-53. doi: 10.1007/s11095-013-1199-9. Epub 2013 Sep 25. Pharm Res. 2014. PMID: 24065593 Review.
-
Discrimination of recombinant from natural human growth hormone using DNA aptamers.J Biomol Tech. 2011 Apr;22(1):27-36. J Biomol Tech. 2011. PMID: 21455479 Free PMC article.
-
UV photodegradation of murine growth hormone: chemical analysis and immunogenicity consequences.Eur J Pharm Biopharm. 2014 Jul;87(2):395-402. doi: 10.1016/j.ejpb.2014.04.005. Epub 2014 Apr 20. Eur J Pharm Biopharm. 2014. PMID: 24758742 Free PMC article.
-
"De-novo" amino acid sequence elucidation of protein G'e by combined "top-down" and "bottom-up" mass spectrometry.J Am Soc Mass Spectrom. 2015 Mar;26(3):482-92. doi: 10.1007/s13361-014-1053-2. Epub 2015 Jan 6. J Am Soc Mass Spectrom. 2015. PMID: 25560987 Free PMC article.
-
Applications in Which Aptamers Are Needed or Wanted in Diagnostics and Therapeutics.Pharmaceuticals (Basel). 2022 Jun 1;15(6):693. doi: 10.3390/ph15060693. Pharmaceuticals (Basel). 2022. PMID: 35745612 Free PMC article. Review.
References
-
- Ahangari G, Ostadali MR, Rabani A, Rashidian J, Sanati MH, Zarindast MR. Growth hormone antibodies formation in patients treated with recombinant human growth hormone. Int J Immunopathol Pharmacol. 2004;17:33–38. - PubMed
-
- Takano K, Shizume K. Current clinical trials with authentic recombinant human growth hormone in Japan. Acta paediatr Scand Suppl. 1986:93–97. - PubMed
-
- Massa G, Vanderschueren-Lodeweyckx M, Buillon R. Five-year follow-up of growth hormone antibodies in growth hormone deficient children treated with recombinant human growth hormone. Clin Endocrinol (Oxf) 1993;38:137–142. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

