The responses of Ht22 cells to oxidative stress induced by buthionine sulfoximine (BSO)
- PMID: 15707499
- PMCID: PMC549549
- DOI: 10.1186/1471-2202-6-10
The responses of Ht22 cells to oxidative stress induced by buthionine sulfoximine (BSO)
Abstract
Background: glutathione (GSH) is the most abundant thiol antioxidant in mammalian cells. It directly reacts with reactive oxygen species (ROS), functions as a cofactor of antioxidant enzymes, and maintains thiol redox potential in cells. GSH depletion has been implicated in the pathogenesis of neurological diseases, particularly to Parkinson's disease (PD). The purpose of this study was to investigate the change of cellular antioxidant status and basic cell functions in the relatively early stages of GSH depletion.
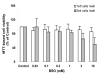
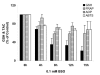
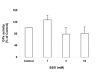
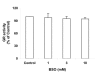
Results: in this study, GSH was depleted by inhibition of glutamylcysteine synthetase using buthionine sulfoximine (BSO) treatment in Ht22, a neuronal cell line derived from mouse hippocampus. Treatment with BSO produced dose-dependent decreases in total GSH level, Fe3+-reducing ability (FRAP assay), Cu2+-reducing ability (Antioxidant Potential, AOP assay), and ABTS free radical scavenging ability (ABTS assay) of the cells, but the sensitivity of these indicators to dosage varied considerably. Most of the changes were completed during the first 8 hours of treatment. Cell viability was tested by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromid) assay, and cells at lower density in culture were found to be more sensitive to GSH depletion. The activity of antioxidant enzymes, such as glutathione peroxidase (GPx), glutathione reductase (GR), and copper/zinc superoxide dismutase (Cu/Zn-SOD) were affected by GSH depletion. A cDNA expression array assay of the effects of BSO treatment showed significantly decreased mRNA level for 3 genes, and significantly increased mRNA level for 10 genes, including the antioxidant enzymes Cu/Zn-SOD and thioredoxin peroxidase 2 (TPxII).
Conclusions: the study suggests that there are BSO-sensitive and BSO-resistant pools of GSH in Ht22 cells, and that different categories of antioxidant react differently to GSH depletion. Further, the effect of GSH status on cell viability is cell density dependent. Finally, the alterations in expression or activity of several antioxidant enzymes provide insight into the various cellular responses to GSH depletion.
Figures





Similar articles
-
Overproduction of Cu/Zn-superoxide dismutase or Bcl-2 prevents the brain mitochondrial respiratory dysfunction induced by glutathione depletion.Exp Neurol. 1999 Aug;158(2):428-36. doi: 10.1006/exnr.1999.7108. Exp Neurol. 1999. PMID: 10415149
-
TRPM2 channel protective properties of N-acetylcysteine on cytosolic glutathione depletion dependent oxidative stress and Ca2+ influx in rat dorsal root ganglion.Physiol Behav. 2012 May 15;106(2):122-8. doi: 10.1016/j.physbeh.2012.01.014. Epub 2012 Jan 24. Physiol Behav. 2012. PMID: 22300897
-
Modulation of rat erythrocyte antioxidant defense system by buthionine sulfoximine and its reversal by glutathione monoester therapy.Biochim Biophys Acta. 2004 Mar 2;1688(2):121-9. doi: 10.1016/j.bbadis.2003.11.004. Biochim Biophys Acta. 2004. PMID: 14990342
-
Cardiovascular and renal manifestations of glutathione depletion induced by buthionine sulfoximine.Am J Hypertens. 2012 Jun;25(6):629-35. doi: 10.1038/ajh.2011.240. Epub 2012 Jan 5. Am J Hypertens. 2012. PMID: 22223042 Review.
-
Glutathione depletion and oxidative stress.Parkinsonism Relat Disord. 2002 Sep;8(6):385-7. doi: 10.1016/s1353-8020(02)00018-4. Parkinsonism Relat Disord. 2002. PMID: 12217624 Review.
Cited by
-
Intracellular hydrogen sulfide induces stress granule formation and translational repression through eIF2α phosphorylation.Arch Toxicol. 2025 Jun;99(6):2419-2431. doi: 10.1007/s00204-025-04026-y. Epub 2025 Apr 9. Arch Toxicol. 2025. PMID: 40202609
-
Bioreducible liposomes for gene delivery: from the formulation to the mechanism of action.PLoS One. 2010 Oct 15;5(10):e13430. doi: 10.1371/journal.pone.0013430. PLoS One. 2010. PMID: 20976172 Free PMC article.
-
N-acetyl-cysteine blunts 6-hydroxydopamine- and L-buthionine-sulfoximine-induced apoptosis in human mesenchymal stromal cells.Mol Biol Rep. 2019 Aug;46(4):4423-4435. doi: 10.1007/s11033-019-04897-2. Epub 2019 May 30. Mol Biol Rep. 2019. PMID: 31147858
-
Protective Effect of Tempol on Buthionine Sulfoximine-Induced Mitochondrial Impairment in Hippocampal Derived HT22 Cells.Oxid Med Cell Longev. 2016;2016:5059043. doi: 10.1155/2016/5059043. Epub 2016 Mar 16. Oxid Med Cell Longev. 2016. PMID: 27069531 Free PMC article.
-
Antioxidant and Antigenotoxic Potential of Infundibulicybe geotropa Mushroom Collected from Northwestern Turkey.Oxid Med Cell Longev. 2020 Feb 19;2020:5620484. doi: 10.1155/2020/5620484. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32148651 Free PMC article.
References
-
- Lockhart B, Jones C, Cuisinier C, Villain N, Peyroulan D, Lestage P. Inhibition of L-homocysteic acid and buthionine sulphoximine-mediated neurotoxicity in rat embryonic neuronal cultures with alpha-lipoic acid enantiomers. Brain Res. 2000;855:292–297. doi: 10.1016/S0006-8993(99)02372-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

