Treatment continuation in patients receiving biological agents or conventional DMARD therapy
- PMID: 15708884
- PMCID: PMC1755655
- DOI: 10.1136/ard.2004.031476
Treatment continuation in patients receiving biological agents or conventional DMARD therapy
Abstract
Objective: To compare drug continuation rates in patients with rheumatoid arthritis who start on a biological agent and in a control group of patients with a change in disease modifying antirheumatic drug (DMARD) treatment after previous DMARD failure.
Methods: Patients with rheumatoid arthritis enrolled in the German biologics register between May 2001 and September 2003 were included in the study. Data were available for 511 patients treated with etanercept, 343 with infliximab, 70 with anakinra, and 599 controls. Propensity scores were used to select a subsample of patients from the control group who were likely to be treated with biological agents because of their disease severity, as well as comparable infliximab and etanercept cases.
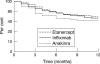
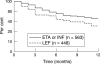
Results: Treatment continuation after 12 months was similar for etanercept (68.6% (95% confidence interval, 62% to 75%)) and infliximab (65.4% (58% to 73%)) but lower for anakinra (59% (41% to 77%)). Treatment continuation was more likely for patients on combinations of biological agents and DMARDs than for those on infliximab or etanercept alone. Patients treated with biological agents were more severely ill than those in the control group and had more previous DMARD failures. After adjustment for baseline differences, the continuation rates were higher in patients treated with biological agents than in comparable control patients treated with leflunomide or leflunomide/methotrexate.
Conclusions: Treatment continuation of biological agents in clinical practice is less likely than in randomised clinical trials but more likely than in comparable controls treated with conventional DMARDs.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical